AMH protects the ovary from doxorubicin by regulating cell fate and the response to DNA damage
- PMID: 39874288
- PMCID: PMC11804487
- DOI: 10.1073/pnas.2414734122
AMH protects the ovary from doxorubicin by regulating cell fate and the response to DNA damage
Abstract
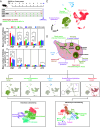
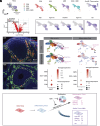
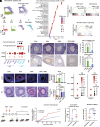
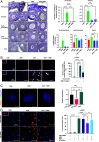
Anti-Müllerian hormone (AMH) protects the ovarian reserve from chemotherapy, and this effect is most pronounced with Doxorubicin (DOX). However, DOX toxicity and AMH rescue mechanisms in the ovary have remained unclear. Herein, we characterize the consequences of these treatments in ovarian cell types using scRNAseq. DOX-induced DNA damage activates Tp53 class mediators across ovarian cell types. In the mesenchyme, cotreatment with AMH halts theca progenitor differentiation and reduces apoptotic gene expression. In preantral granulosa cells, DOX upregulates the cell cycle inhibitor Cdkn1a and dysregulates Wnt signaling, which are ameliorated by AMH cotreatment. Finally, AMH induces Id3, a gene involved in DNA repair, which is necessary to prevent the accumulation of DNA lesions marked by γ-H2AX. Altogether these mechanisms of AMH protection contribute to sustained fertility in mice, offering promising broad avenues for fertility preservation in cancer patients undergoing chemotherapy.
Keywords: AMH; chemotherapy; oncofertility; ovary.
Conflict of interest statement
Competing interests statement:Scientific advisors and co-founders of Oviva Therapeutics (P.K.D. and D.P.). Stock ownership in Oviva Therapeutics (P.K.D. and D.P.). PCT/US2017/066346–WO2018112168–Müllerian inhibiting substance (MIS) proteins for ovarian and uterine oncoprotection, and ovarian reserve and uterine preservation (P.K.D. and D.P.).
Figures

Update of
-
AMH protects the ovary from doxorubicin by regulating cell fate and the response to DNA damage.bioRxiv [Preprint]. 2024 May 23:2024.05.23.595356. doi: 10.1101/2024.05.23.595356. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Feb 04;122(5):e2414734122. doi: 10.1073/pnas.2414734122. PMID: 38826466 Free PMC article. Updated. Preprint.
References
-
- Demeestere I., et al. , No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: Final long-term report of a prospective randomized trial. J. Clin. Oncol. 34, 2568–2574 (2016). - PubMed
-
- Lambertini M., et al. , Debated role of ovarian protection with gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in women with cancer. J. Clin. Oncol. 35, 804–805 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

