Outcome of high-grade B-cell lymphoma compared with other large B-cell lymphoma after CAR-T rescue: a DESCAR-T LYSA study
- PMID: 39874518
- PMCID: PMC12148394
- DOI: 10.1182/bloodadvances.2024014732
Outcome of high-grade B-cell lymphoma compared with other large B-cell lymphoma after CAR-T rescue: a DESCAR-T LYSA study
Abstract
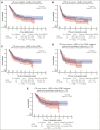
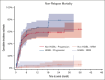
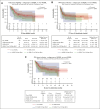
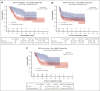
High-grade B-cell lymphoma (HGBL) with MYC and BCL2 and/or BCL6 rearrangements (double hit [HGBL-DH] or triple hit [HGBL-TH]) or not otherwise specified (HGBL-NOS) are considered to be more aggressive diseases among large B-cell lymphomas (LBCLs). CD19-targeting chimeric antigen receptor (CAR) T cells have changed the prognosis of chemoresistant LBCL. Clinical and pathological data of patients treated for relapsed/refractory LBCL or HGBL in third line or more, all characterized by fluorescence in situ hybridization, were collected from the French DESCAR-T registry. Between January 2018 and November 2022, a total of 228 patients were included across 14 centers, 73 with HGBL (28 HGBL-DH MYC-BCL2, 14 HGBL-TH, 8 HGBL-DH MYC-BCL6, and 23 HGBL-NOS) and 155 with non-HGBL. The median follow-up was 18.5 months (95% confidence interval [CI], 14.3-23.4) from the date of infusion. Progression-free survival and overall survival (OS) were not significantly different between HGBL and non-HGBL, at 3.2 months (95% CI, 2.8-6.0) vs 4.5 months (95% CI, 3.1-8.7; P = .103) and 15.4 months (95% CI, 5.6-32.4) vs 18.3 months (95% CI, 8.5 to not reached), respectively. From the date of eligibility, the median OS was inferior for patients with HGBL-TH/DH MYC-BCL2 at 6.6 months vs 18.5 months for HGBL-NOS vs 13.6 months for HGBL-DH MYC-BCL6 vs 11.8 months for LBCL (P = .037). However, patients who received infusion presented the same outcome. CAR T-cell therapy used in third line or more seems to overcome the poor prognosis of HGBL subtypes, especially in HGBL-TH/DH MYC-BCL2. This observation supports considering the potential benefit of using CAR T cells earlier in disease course.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.B. received honoraria from Kite, a Gilead Company, and Novartis. F.-X.G. received consulting fees and travel and accommodation expenses from Kite/Gilead and Novartis. R.D.B. received honoraria from Kite, a Gilead Company, and Novartis. O.C. received honoraria from Kite, a Gilead Company. F.J. received honoraria from Gilead. M.M. received honoraria from Kite, a Gilead company, and Novartis. L.R. received travel grants from Kite/Gilead. The remaining authors declare no competing financial interests.
Figures

References
-
- Landsburg DJ, Ayers EC, Bond DA, et al. Poor outcomes for double-hit lymphoma patients treated with curative-intent second-line immunochemotherapy following failure of intensive front-line immunochemotherapy. Br J Haematol. 2020;189(2):313–317. - PubMed
-
- Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol. 2014;166(6):891–901. - PubMed
-
- Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280–288. - PubMed
-
- Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319–2331. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

