The role of nutritional vitamin D in chronic kidney disease-mineral and bone disorder in children and adults with chronic kidney disease, on dialysis, and after kidney transplantation-a European consensus statement
- PMID: 39875204
- PMCID: PMC11960744
- DOI: 10.1093/ndt/gfae293
The role of nutritional vitamin D in chronic kidney disease-mineral and bone disorder in children and adults with chronic kidney disease, on dialysis, and after kidney transplantation-a European consensus statement
Abstract
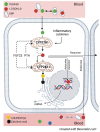
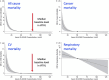
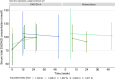
Vitamin D deficiency is common in patients with chronic kidney disease (CKD) and associates with poor outcomes. Current clinical practice guidelines recommend supplementation with nutritional vitamin D as for the general population. However, recent large-scale clinical trials in the general population failed to demonstrate a benefit of vitamin D supplementation on skeletal or non-skeletal outcomes, fueling a debate on the rationale for screening for and correcting vitamin D deficiency, both in non-CKD and CKD populations. In a collaboration between the European Renal Osteodystrophy initiative of the European Renal Association (ERA) and the European Society for Paediatric Nephrology (ESPN), an expert panel performed an extensive literature review and formulated clinical practice points on vitamin D supplementation in children and adults with CKD and after kidney transplantation. These were reviewed by a Delphi panel of members from relevant working groups of the ERA and ESPN. Key clinical practice points include recommendations to monitor for, and correct, vitamin D deficiency in children and adults with CKD and after kidney transplantation, targeting 25-hydroxyvitamin D levels >75 nmol/l (>30 ng/ml). Although vitamin D supplementation appears well-tolerated and safe, it is recommended to avoid mega-doses (≥100 000 IU) and very high levels of 25 hydroxyvitamin D (>150-200 nmol/l, or 60-80 ng/ml) to reduce the risk of toxicity. Future clinical trials should investigate the benefit of vitamin D supplementation on patient-relevant outcomes in the setting of vitamin D deficiency across different stages of CKD.
Keywords: chronic kidney disease–mineral and bone disorder; kidney transplantation; parathyroid hormone; renal osteodystrophy; vitamin D.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
J. Bacchetta reports support from Viatris/Mylan; J. Bover reports support from Abbvie, Amgen, Astra Zeneca, CSL Vifor, GSK, Rubió and Sanofi; M. de Borst reports support from Amgen, Astra Zeneca, Bayer, CSL Vifor, Kyowa Kiri Pharma, Pharmacosmos, and Sanofi Genzyme; E. Cavalier reports support from Fujirebio, IDS and Roche Diagnostics; M. Cozzolino reports support from Amgen and Vifor CSL; A.C. Ferreira report support from Astra Zeneca, Bayer CSL and Vifor Pharma; D. Hansen reports support from Astra Zeneca, Gedeon Richter, GSK, UCB Nordic and VIFOR pharma; M. Herrmann reports support from Roche Diagnostics, Sanofi, Shimadzu and Sysemx; H.S. Jørgensen reports support from Abiogen Pharma; S. Mazzaferro reports support from Amgen; R. Shroff reports support from Amgen, Astra Zeneca, Fresenius Medical Care and Vitaflo; M. Vervloet reports support from Astra Zeneca, Boehringer Ingelheim, Fresenius Medical Care, Medice and Vifor Pharma; R. de Jongh and M. Wan have no disclosures.
Figures




References
-
- J-DAVID Investigators , Shoji T, Inaba M, Fukagawa M et al. Effect of oral alfacalcidol on clinical outcomes in patients without secondary hyperparathyroidism receiving maintenance hemodialysis: the J-DAVID randomized clinical trial. JAMA 2018;320:2325–34. 10.1001/jama.2018.17749 - DOI - PMC - PubMed

