Partially Bonded Crystals: A Pathway to Porosity and Polymorphism
- PMID: 39875319
- PMCID: PMC11823632
- DOI: 10.1021/acsnano.4c06489
Partially Bonded Crystals: A Pathway to Porosity and Polymorphism
Abstract
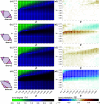
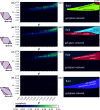
In recent years, experimental and theoretical investigations have shown that anisotropic colloids can self-organize into ordered porous monolayers, where the interplay of localized bonding sites, so-called patches, with the particle's shape is responsible for driving the systems away from close-packing and toward porosity. Until now it has been assumed that patchy particles have to be fully bonded with their neighboring particles for crystals to form, and that, if full bonding cannot be achieved due to the choice of patch placement, disordered assemblies will form instead. In contrast, we show that by deliberately displacing the patches such that full bonding is disfavored, a different route to porous crystalline monolayers emerges, where geometric frustration and partial bonding are decisive process. The resulting dangling bonds lead to the emergence of effectively chiral units which then act as building blocks for energetically equivalent crystal polymorphs.
Keywords: frustration; patchy colloids; polymorphism; self-assembly; shape-anisotropy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
A Matter of Size and Placement: Varying the Patch Size of Anisotropic Patchy Colloids.Int J Mol Sci. 2020 Nov 16;21(22):8621. doi: 10.3390/ijms21228621. Int J Mol Sci. 2020. PMID: 33207624 Free PMC article.
-
Self-assembly and phase behavior of mixed patchy colloids with any bonding site geometry: theory and simulation.Soft Matter. 2020 Apr 15;16(15):3806-3820. doi: 10.1039/d0sm00248h. Soft Matter. 2020. PMID: 32242603
-
Patchy colloids: state of the art and perspectives.Phys Chem Chem Phys. 2011 Apr 14;13(14):6397-410. doi: 10.1039/c0cp02296a. Epub 2011 Feb 18. Phys Chem Chem Phys. 2011. PMID: 21331432
-
Designing Hydrogen-Bonded Organic Frameworks (HOFs) with Permanent Porosity.Angew Chem Int Ed Engl. 2019 Aug 12;58(33):11160-11170. doi: 10.1002/anie.201902147. Epub 2019 May 17. Angew Chem Int Ed Engl. 2019. PMID: 30891889 Review.
-
Design and Preparation of Nematic Colloidal Particles.Langmuir. 2022 Aug 2;38(30):9099-9118. doi: 10.1021/acs.langmuir.2c00611. Epub 2022 Jul 22. Langmuir. 2022. PMID: 35866261 Review.
References
-
- Sacanna S.; Pine D. J. Shape-anisotropic colloids: Building blocks for complex assemblies. Curr. Opin. Colloid Interface Sci. 2011, 16, 96–105. 10.1016/j.cocis.2011.01.003. - DOI
LinkOut - more resources
Full Text Sources

