Neural circuits underlying context-dependent competition between defensive actions in Drosophila larvae
- PMID: 39875414
- PMCID: PMC11775277
- DOI: 10.1038/s41467-025-56185-2
Neural circuits underlying context-dependent competition between defensive actions in Drosophila larvae
Abstract
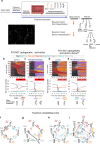
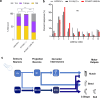
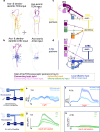
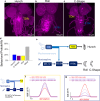
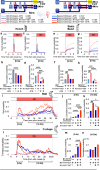
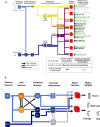
To ensure their survival, animals must be able to respond adaptively to threats within their environment. However, the precise neural circuit mechanisms that underlie flexible defensive behaviors remain poorly understood. Using neuronal manipulations, machine learning-based behavioral detection, electron microscopy (EM) connectomics and calcium imaging in Drosophila larvae, we map second-order interneurons that are differentially involved in the competition between defensive actions in response to competing aversive cues. We find that mechanosensory stimulation inhibits escape behaviors in favor of startle behaviors by influencing the activity of escape-promoting second-order interneurons. Stronger activation of those neurons inhibits startle-like behaviors. This suggests that competition between startle and escape behaviors occurs at the level of second-order interneurons. Finally, we identify a pair of descending neurons that promote startle behaviors and could modulate the escape sequence. Taken together, these results characterize the pathways involved in startle and escape competition, which is modulated by the sensory context.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- von Reyn, C. R. et al. A spike-timing mechanism for action selection. Nat. Neurosci.17, 962–970 (2014). - PubMed
MeSH terms
Grants and funding
- ANR-17-CE37-0019-01/Agence Nationale de la Recherche (French National Research Agency)
- ANR-22-CE37-0027/Agence Nationale de la Recherche (French National Research Agency)
- ANR-20-IDEES-0002/Agence Nationale de la Recherche (French National Research Agency)
- ANR-17-CE23-0016/Agence Nationale de la Recherche (French National Research Agency)
- PIA/ANR-16-CONV-0005,OG/Agence Nationale de la Recherche (French National Research Agency)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

