A morphometric signature to identify ductal carcinoma in situ with a low risk of progression
- PMID: 39875514
- PMCID: PMC11775207
- DOI: 10.1038/s41698-024-00769-6
A morphometric signature to identify ductal carcinoma in situ with a low risk of progression
Abstract
Ductal carcinoma in situ (DCIS) may progress to ipsilateral invasive breast cancer (iIBC), but often never will. Because DCIS is treated as early breast cancer, many women with harmless DCIS face overtreatment. To identify features associated with progression, we developed an artificial intelligence-based DCIS morphometric analysis pipeline (AIDmap) on hematoxylin-eosin-stained (H&E) tissue sections. We analyzed 689 digitized H&Es of pure primary DCIS of which 226 were diagnosed with subsequent iIBC and 463 were not. The distribution of 15 duct morphological measurements was summarized in 55 morphometric variables. A ridge regression classifier with cross validation predicted 5-years-free of iIBC with an area-under the curve of 0.67 (95% CI 0.57-0.77). A combined clinical-morphometric signature, characterized by small-sized ducts, a low number of cells and a low DCIS/stroma ratio, was associated with outcome (HR = 0.56; 95% CI 0.28-0.78). AIDmap has potential to identify harmless DCIS that may not need treatment.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

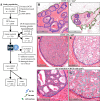
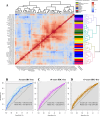



Update of
-
Artificial intelligence-based morphometric signature to identify ductal carcinoma in situ with low risk of progression to invasive breast cancer.Res Sq [Preprint]. 2023 Dec 13:rs.3.rs-3639521. doi: 10.21203/rs.3.rs-3639521/v1. Res Sq. 2023. Update in: NPJ Precis Oncol. 2025 Jan 28;9(1):25. doi: 10.1038/s41698-024-00769-6. PMID: 38168198 Free PMC article. Updated. Preprint.
References
-
- Ringberg, A., Palmer, B., Linell, F., Rychterova, V. & Ljungberg, O. Bilateral and multifocal breast carcinoma. A clinical and autopsy study with special emphasis on carcinoma in situ. Eur. J. Surg. Oncol17, 20–29 (1991). - PubMed
-
- Maxwell, A. J. et al. Risk factors for the development of invasive cancer in unresected ductal carcinoma in situ. Eur. J. Surg. Oncol.44, 429–435 (2018). - PubMed
-
- Myers, E. R. et al. Benefits and harms of breast cancer screening: a systematic review. JAMA314, 1615–1634 (2015). - PubMed
-
- Falk, R. S., Hofvind, S., Skaane, P. & Haldorsen, T. Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast cancer Res. Treat.129, 929–938 (2011). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

