Raptin, a sleep-induced hypothalamic hormone, suppresses appetite and obesity
- PMID: 39875551
- PMCID: PMC11909135
- DOI: 10.1038/s41422-025-01078-8
Raptin, a sleep-induced hypothalamic hormone, suppresses appetite and obesity
Erratum in
-
Author Correction: Raptin, a sleep-induced hypothalamic hormone, suppresses appetite and obesity.Cell Res. 2025 Jul;35(7):530-531. doi: 10.1038/s41422-025-01123-6. Cell Res. 2025. PMID: 40328926 Free PMC article. No abstract available.
Abstract
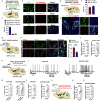
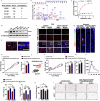
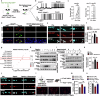
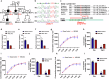
Sleep deficiency is associated with obesity, but the mechanisms underlying this connection remain unclear. Here, we identify a sleep-inducible hypothalamic protein hormone in humans and mice that suppresses obesity. This hormone is cleaved from reticulocalbin-2 (RCN2), and we name it Raptin. Raptin release is timed by the circuit from vasopressin-expressing neurons in the suprachiasmatic nucleus to RCN2-positive neurons in the paraventricular nucleus. Raptin levels peak during sleep, which is blunted by sleep deficiency. Raptin binds to glutamate metabotropic receptor 3 (GRM3) in neurons of the hypothalamus and stomach to inhibit appetite and gastric emptying, respectively. Raptin-GRM3 signaling mediates anorexigenic effects via PI3K-AKT signaling. Of note, we verify the connections between deficiencies in the sleeping state, impaired Raptin release, and obesity in patients with sleep deficiency. Moreover, humans carrying an RCN2 nonsense variant present with night eating syndrome and obesity. These data define a unique hormone that suppresses food intake and prevents obesity.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- 92149306/National Natural Science Foundation of China (National Science Foundation of China)
- 82120108009/National Natural Science Foundation of China (National Science Foundation of China)
- 81930022/National Natural Science Foundation of China (National Science Foundation of China)
- 82201746/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

