Efficacy and Safety of Boswellia serrata and Apium graveolens L. Extract Against Knee Osteoarthritis and Cartilage Degeneration: A Randomized, Double-blind, Multicenter, Placebo-Controlled Clinical Trial
- PMID: 39875757
- PMCID: PMC11880083
- DOI: 10.1007/s11095-025-03818-2
Efficacy and Safety of Boswellia serrata and Apium graveolens L. Extract Against Knee Osteoarthritis and Cartilage Degeneration: A Randomized, Double-blind, Multicenter, Placebo-Controlled Clinical Trial
Abstract
Background: Osteoarthritis is the prevailing form of inflammatory condition in joints of adults and the aging population, leading to long-term disability and chronic pain. Current therapeutic options have variable therapeutic efficacy and/or several side effects.
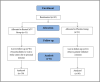
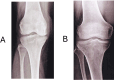
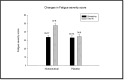
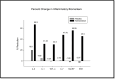
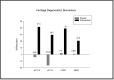
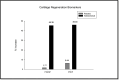
Methods: A randomized, placebo-controlled, double-blind clinical trial was conducted in 62 participants using a nutraceutical [standardized Boswellia serrata Roxb. gum resin (300 mg) and Apium graveolens L. seed extract (250 mg)], to determine its safety and efficacy for supporting cartilage health and reduction in knee osteoarthritis symptoms. All participants were assessed for physical function and pain with the help of WOMAC, VAS, Physicians' Global Assessment for the six-minute walk test/pain. Knee X-ray, KOOS questionnaire score, and FACIT-F score were assessed. Additionally, inflammatory, cartilage degeneration and regeneration biomarkers in serum and urine were evaluated at baseline and after 90 days of treatment.
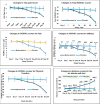
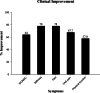
Results: Oral administration of the nutraceutical resulted in prolonged symptomatic relief with reduced pain, stiffness, and swelling. Inflammatory (serum IL-7, IL-1, IL-6, hs-CRP, TNF-α, ESR) and cartilage degeneration biomarkers (serum CTX-II, COMP, MMP-3 and urinary CTX-II) were decreased in the nutraceutical group compared to baseline and placebo. Furthermore, serum N-propeptide of collagen IIA (PIIANP) and procollagen-type-C propeptide (PIICP) levels were increased in the nutraceutical group, suggesting collagen synthesis contributing to cartilage regeneration. At given doses for 90 days, there were no adverse effects based on the clinical examination, biochemical, hematological, and ECG analysis.
Conclusions: Taken together, the combination of Boswellia and celery could be a safe and promising herbal nutraceutical option for managing osteoarthritis and cartilage health effectively.
Keywords: cartilage support; clinical trial; inflammation; knee osteoarthritis; nutraceutical.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: RestorCel™ is marketed by Phytoveda Pvt. Ltd., Mumbai, India which funded this study. CG, AM, DM and SN are employees of Phytoveda Pvt. Ltd., Mumbai, India and Viridis Biopharma Pvt. Ltd., Mumbai, India. However, these authors had no role in the conduct of the trial or analysis of results. The clinical trial was conducted by an external Contract Research Organisation (CRO), MPREX Healthcare Pvt. Ltd., Pune, India, who analyzed the results themselves along with the clinicians conducting the study in this multicentric trial (NV, RA, DGD, HP, GG, DN).
Figures
References
-
- Ohio CV PharmD, BCPS Assistant Professor of Pharmacy Practice University of Findlay Findlay, Ohio Laura A Perry, PharmD, BCPS Professor of Pharmacy Practice/ University of Findlay Findlay, Ohio Amanda Crase, PharmD Candidate 2024 University of Findlay Findlay, Ohio Anthony Swierz, PharmD Candidate 2024 University of Findlay Findlay, Ohio Sydney Wenzinger, PharmD Candidate 2024 University of Findlay Findlay. Guiding Osteoarthritis Management. [cited 2024 Aug 17]. Available from: https://www.uspharmacist.com/article/guiding-osteoarthritis-management.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

