Oestrogen suppresses the adipogenesis of fibro/adipogenic progenitors through reactivating the METTL3-ESR1-mediated loop in post-menopausal females
- PMID: 39875775
- PMCID: PMC11774659
- DOI: 10.1002/ctm2.70206
Oestrogen suppresses the adipogenesis of fibro/adipogenic progenitors through reactivating the METTL3-ESR1-mediated loop in post-menopausal females
Abstract
Background: Post-menopausal women experience more severe muscular fatty infiltration, though the mechanisms remain unclear. The decline in estrogen levels is considered as a critical physiological alteration during post-menopause. Fibro/adipogenic progenitors (FAPs) are identified as major contributors to muscular fatty infiltration. This study aimed to investigate the detailed mechanism underlying the excessive muscular fatty infiltration in postmenopausal females.
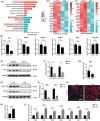
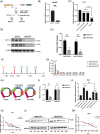
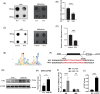
Methods: Supraspinatus muscle samples were collected from female patients with or without menopause, and from mice with or without ovariectomy (OVX), to evaluate muscular fatty infiltration and isolated FAPs. The expressions of (estrogen receptor 1) ESR1, methyltransferase-like 3 (METTL3), and adipogenesis ability in FAPs from post-menopausal women and OVX mice were investigated. RNA sequencing (RNA-Seq) was performed to explore the gene expression profiles and potential mechanisms in FAPs from Pdgfrα-CreERT2; Esr1 knockout (Esr1 KO) mice and Esr1 flox/flox (Esr1 f/f) mice. The interplay of the METTL3-ESR1 mediated loop and its role in regulating adipogenesis in FAPs were investigated using dual luciferase reporter assays, chromatin immunoprecipitation (ChIP), and protein and RNA stability assays. The effects of estrogen supplementation on muscular fatty infiltration and locomotor function in OVX mice were evaluated by immunofluorescent staining and functional analysis.
Results: Decreased expression of ESR1/METTL3 and increased adipogenesis ability in FAPs was found in post-menopausal female. METTL3-mediated m6A methylation promoted ESR1 mRNA stability at the post-transcriptional level in FAPs. METTL3-mediated m6A modification promoted ESR1 expression by stabilizing ESR1 mRNA, while ESR1 acted as a transcription factor that enhanced METTL3 transcription in turn. ESR1 also suppressed the transcription of the adipogenic transcription factor peroxisome proliferator-activated receptor gamma (PPARγ), thereby inhibiting adipogenesis in FAPs. Reactivation of the METTL3-ESR1 mediated loop by estrogen alleviated excessive adipogenesis in FAPs from post-menopausal women, and it also reduced muscular fatty infiltration, and improved locomotor function in OVX mice.
Conclusion: Excessive muscular fatty infiltration in post-menopausal women arose from the disruption of the METTL3-ESR1 mediated loop of FAPs due to estrogen deficiency. Reactivation of the METTL3-ESR1 mediated loop by estrogen may serve as a novel intervention to inhibit excessive adipogenesis of post-menopausal female FAPs, thereby ameliorating muscular fatty infiltration and improving locomotor function in post-menopausal females.
Key points: Oestrogen insufficiency disrupted the METTL3ESR1 loop in post-menopausal FAPs, causing excessive muscular fatty infiltration. METTL3-mediated m6A modification stabilized ESR1 mRNA and enhanced ESR1 expression, while increased ESR1 further promoted METTL3 transcription. ESR1 inhibited the transcription of adipogenic factor PPARγ, ameliorating adipogenesis in FAPs. Reactivating the METTL3ESR1 loop via oestrogen in FAPs reduced muscular fatty infiltration and improved locomotor function.
Keywords: ESR1; METTL3; adipogenesis; fibro/adipogenic progenitors (FAPs); m6A methylation; muscular fatty infiltration.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Vitale JA, Messina C, Albano D, et al. Appendicular muscle mass, thigh intermuscular fat infiltration, and risk of fall in postmenopausal osteoporotic elder women. Gerontology. 2021;67(4):415‐424. - PubMed
-
- Yuri T, Nankaku M, Kawano T, et al. Evaluating the contribution of fat infiltration in anterior gluteus minimus muscle to walking ability in female with unilateral hip osteoarthritis and candidates for total hip arthroplasty. Clin Biomech (Bristol, Avon). 2023;103:105909. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
