Barriers to and facilitators of healthcare professionals in ADR reporting in a tertiary care hospital in India
- PMID: 39875957
- PMCID: PMC11773872
- DOI: 10.1186/s12913-024-12139-w
Barriers to and facilitators of healthcare professionals in ADR reporting in a tertiary care hospital in India
Abstract
Introduction: Several adverse drug reactions (ADRs) go unreported within a healthcare setting despite the risks they cause. We therefore decided to conduct this study in order to recognize the obstacles that hinder the healthcare professionals (HCPs) in a tertiary care hospital in Kattankulathur, Tamil Nadu from reporting ADRs and what strategies ought to be implemented.
Methods: We carried out a cross-sectional study among the HCPs such as doctors, pharmacists and nurses within our institution. A pre-validated questionnaire was used to collect data on the socio-demographics, barriers and facilitators in reporting ADR. A 2 weeks timeline was given to the HCPs to fill the questionnaire forms. Out of the 107 forms distributed, we received 80 of them that were duly filled. Data was analyzed using IBM SPSS version 26.
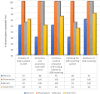
Results: Out of the 80 HCPs, only 22 of them had reported any ADRs in their career. 52% of our HCPs reported the lack of understanding of ADR reporting mechanism as their main hindrance. Additionally, 25 (31%) of the HCPs stated that reporting ADRs is time consuming. 18 (22%) of them reported a fear of legal liability. 13 (16%) of them stated that the reporting from is complicated and 29 (36%) stated a lack of motivation as the reason for not reporting ADR. Majority of our HCPs 76 (95%) recommended the need for continuous medical education and training as the best strategy to improve ADR reporting.
Conclusion: Barriers such as time constraints, workload pressures and competing priorities often hinder HCPs from dedicating adequate attention to ADR reporting. The inclusion of topics related to ADR reporting in the curriculum (i.e. clinical pharmacology) and increased awareness from the ADR monitoring centre were seen to be significant facilitators to enhance ADR reporting among health care practitioners.
Keywords: Adverse drug reaction; Clinical pharmacy; Nurses; Pharmacovigilance; Physicians.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol was approved by the Institutional Ethics Committee of SRM Medical College Hospital and Research Centre, SRM ISR, Kattankulathur, Tamil Nadu, India (Ref No: SRMIEC-ST0923-630). Written informed consent was obtained from the study participants and the study was conducted according to the revised National Ethical Guidelines for Biomedical and Health Research Involving Human Participants, Indian Council of Medical Research (ICMR) 2017. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- Craigle V. MedWatch: The FDA Safety Information and Adverse Event Reporting Program. J Med Libr Assoc. 2007;95(2):224–5. 10.3163/1536-5050.95.2.224. PMCID:PMC1852611. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials