Steatohepatitis-induced vascular niche alterations promote melanoma metastasis
- PMID: 39875968
- PMCID: PMC11776123
- DOI: 10.1186/s40170-025-00374-6
Steatohepatitis-induced vascular niche alterations promote melanoma metastasis
Abstract
Background: In malignant melanoma, liver metastases significantly reduce survival, even despite highly effective new therapies. Given the increase in metabolic liver diseases such as metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH), this study investigated the impact of liver sinusoidal endothelial cell (LSEC)-specific alterations in MASLD/MASH on hepatic melanoma metastasis.
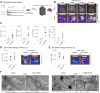
Methods: Mice were fed a choline-deficient L-amino acid-defined (CDAA) diet for ten weeks to induce MASH-associated liver fibrosis, or a CDAA diet or a high fat diet (HFD) for shorter periods of time to induce early steatosis-associated alterations. Liver metastasis formation was assessed using melanoma cell lines B16F10Luc2 and Wt31. LSEC-specific GATA4 knockout mice (Gata4LSEC-KO/BL) developing MASH-like liver fibrosis without steatosis via a pathogenic angiocrine switch were included to compare the impact of liver fibrosis versus hepatic steatosis on hepatic melanoma metastasis. Bulk RNA-Seq of isolated LSECs from CDAA-fed and control mice was performed. Levels of adhesion molecules (VCAM1, ICAM1, E-selectin) were monitored, and ICAM1 and VCAM1 antibody therapy was employed.
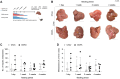
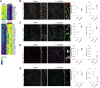
Results: Feeding a CDAA diet, in contrast to a HFD, led to increased metastasis before the development of liver fibrosis. Gata4LSEC-KO/BL mice characterized by vascular changes ensuing perisinusoidal liver fibrosis without steatosis also exhibited increased metastasis. Early molecular alterations in the hepatic vascular niche, rather than fibrosis or steatosis, correlated with metastasis, as shown by LSEC dedifferentiation and upregulation of endothelial adhesion molecules. The metastatic process in CDAA-fed mice was also dependent on the respective melanoma cell lines used and on the route of their metastatic spread. ICAM1 inhibition, but not VCAM1 inhibition reduced melanoma cell retention.
Conclusion: We discovered that the hepatic vascular niche acts as a delicate sensor to even short-term nutritional alterations during the development of MASLD/MASH. The dynamic adaptations to the metabolic challenges of developing MASLD/MASH caused an early shift from the normal hepatic vascular niche to a pre-metastatic vascular niche that promoted hepatic melanoma metastasis in the context of cell-autonomous and acquired melanoma cell features. Altogether, our findings provide a potential avenue for angiotargeted therapies to prevent hepatic melanoma metastasis.
Keywords: Cutaneous malignant melanoma; Early vascular alterations; Hepatic metastasis; Liver sinusoidal endothelial cells; Metabolic dysfunction-associated steatohepatitis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences and the animal ethics committee of Baden Wuerttemberg (Regierungspraesidium Karlsruhe) approved all animal experiments. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures





References
-
- Garbe C, Peris K, Hauschild A, Saiag P, Middleton M, Spatz A, et al. Diagnosis and treatment of melanoma. European consensus-based interdisciplinary guideline--Update 2012. Eur J Cancer. 2012;48:2375–90. - PubMed
-
- Belhocine TZ, Scott AM, Even-Sapir E, Urbain JL, Essner R. Role of nuclear medicine in the management of cutaneous malignant melanoma. J Nucl Med. 2006;47:957–67. - PubMed
-
- Patel JK, Didolkar MS, Pickren JW, Moore RH. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–10. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

