Practical management of adverse events in patients receiving tarlatamab, a delta-like ligand 3-targeted bispecific T-cell engager immunotherapy, for previously treated small cell lung cancer
- PMID: 39876075
- PMCID: PMC11775405
- DOI: 10.1002/cncr.35738
Practical management of adverse events in patients receiving tarlatamab, a delta-like ligand 3-targeted bispecific T-cell engager immunotherapy, for previously treated small cell lung cancer
Abstract
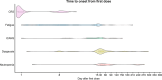
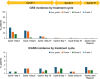
Tarlatamab is a bispecific T-cell engager immunotherapy targeting delta-like ligand 3 (DLL3) and the cluster of differentiation 3 (CD3) molecule. In the phase 2 DeLLphi-301 trial of tarlatamab for patients with previously treated small cell lung cancer, tarlatamab 10 mg every 2 weeks achieved durable responses and encouraging survival outcomes. Analyses of updated safety data from the DeLLphi-301 trial demonstrated that the most common treatment-emergent adverse events were cytokine release syndrome (53%), pyrexia (38%), decreased appetite (36%), dysgeusia (32%), and an emia (30%). Cytokine release syndrome was mostly grade 1 or 2 in severity, occurred primarily after the first or second tarlatamab dose, and was managed with supportive care, which included the administration of antipyretics (e.g., acetaminophen), intravenous hydration, and/or glucocorticoids. Other treatment-emergent adverse effects of interest included neutropenia (16%) and immune effector cell-associated neurotoxicity syndrome and associated neurologic events (10%). Given that tarlatamab is the first T-cell engager approved for the treatment of small cell lung cancer, raising awareness with regard to the monitoring and management of tarlatamab-associated adverse events is essential. Here, the authors describe the timing, occurrence, and duration of these adverse events and review the management and risk-mitigation strategies used by clinical investigators during the DeLLphi-301 trial.
Keywords: DeLLphi‐301; adverse event management; safety profile; tarlatamab.
© 2025 The Author(s). Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Jacob M. Sands reports research funding from Amgen and Harpoon; personal/consulting fees from Amgen Inc., AstraZeneca, Boehringer Ingelheim, AbbVie, Daiichi Sankyo/UCB Japan, Eli Lilly & Company; G1 Therapeutics, Gilead Sciences, Guardant Health, Jazz Pharmaceuticals Inc., Medtronic Inc., PharmaMar, and Sanofi; honoraria from Pfizer; and support for travel/accommodation and expenses from AstraZeneca outside the submitted work. Stéphane Champiat reports institutional research funding from AbbVie, AdaptImmune, Adura Biotech, Agios, Amgen, arGEN‐X BVBA, Arno Therapeutics, Astex Pharmaceuticals, AstraZeneca, Bayer, BB Biotech Ventures, BeiGene, BioAlliance Pharma, BioNTech, Blueprint Medicines, Boehringer Ingelheim, Bolt Biotherapeutics, Boston Pharmaceuticals, Bristol Myers Squibb, Celgene, Centessa Pharmaceuticals, Cephalon, Chugai Pharma, Clovis Oncology, Cullinan Oncology, Cytovation, Daiichi Sankyo, Debiopharm Group, Eisai, Eli Lilly & Company, Exelixis, FORMA Therapeutics, Genentech, Gilead Sciences, GlaxoSmithKline, Glenmark, H3 Biomedicine, Roche, ImCheck Therapeutics, Immunocore, Incyte, Innate Pharma, Janssen‐Cilag, Kura Oncology, Kyowa Hakko Krin, Loxo, Lytix Biopharma, MedImmune, Menarini, Merck KGaA, Merck Sharp & Dohme, Merrimack, Merus, Millennium, Molecular Partners, Nanobiotix, Nektar, Nerviano Medical Sciences, Novartis, Octimet, Oncoethix, OncoMed, Oncopeptides, Onyx, Orion, Oryzon Genomics, OSE Immunotherapeutics, Pfizer, PharmaMar, Philogen, Pierre Fabre, Plexxikon, Replimune, RigonTEC, Roche/Genentech, Sanofi/Aventis, Seagen, SERVIER, Sierra Oncology, Sotio, Syros Pharmaceuticals, Taiho Pharmaceutical, Tesaro, Tioma Therapeutics, Transgene, Turning Point Therapeutics, Vericyte, Wyeth, Y's Therapeutics, and Zencor; other institutional support from AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly & Company, GlaxoSmithKline, Johnson & Johnson, Medimmune, Merck, NH TherAguix, Pfizer, and Roche; honoraria from Amgen, Astellas Pharma, AstraZeneca, Bristol Myers Squibb, Eisai Europe, Fresenius Kabi, Genmab, Janssen Pharmaceuticals, Merck KGaA, Merck Serono, Merck Sharp & Dohme, Novartis, Roche, SERVIER, and Takeda Oncology; personal/consulting or advisory fees from Alderaan Biotechnology, Amgen, AstraZeneca, Avacta Life Sciences, Bayer, BioNTech SE, Celanese, Computen, Domain Therapeutics, Ellipses Pharma, Genmab, Immunicom, Mariana Oncology, Mima Health, Nanobiotix, NextCure, Oncovita, Pierre Fabre, Seagen, Takeda Oncology, Tatum Bioscience, Tollys, and UltraHuman8; patents, royalties, and other intellectual property for patent number WO2010039223A2 (T‐cell immunogens derived from antiviral proteins and methods of using same); support for travel/accommodations and expenses from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Merck, OSE Immunotherapeutics, Roche, and Sotio; and owns stock options in Avacta Life Sciences outside the submitted work. Horst‐Dieter Hummel reports institutional support for professional activities from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb/Pfizer, Johnson & Johnson/Janssen, Merck, and Revolution Medicines; personal/consulting or advisory fees from Amgen and Boehringer Ingelheim; honoraria from Amgen; and support for travel/accommodations and expenses from Amgen and Boehringer Ingelheim outside the submitted work. Hossein Borghaei reports grants/contracts from Amgen; institutional research funding from Amgen, Bristol Myers Squibb, and Eli Lilly & Company; personal/consulting or advisory fees from AbbVie, Amgen, AstraZeneca, Bayer, BeiGene USA Inc., BerGenBio, BioNTech, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo/UCB Japan, Eli Lilly & Company, EMD Serono, Genentech, Genmab, Grid Therapeutics, Guardant Health, IO Biotech, ITeos Therapeutics, IO Biotech, Janssen Oncology, Jazz Pharmaceuticals, Merck KGaA, Merck Sharp & Dohme, Mirati Therapeutics, Natera, Novartis, Novocure, Oncocyte, Pfizer, PharmaMar, Puma Biotechnology, RAPT Therapeutics, Regeneron Pharmaceuticals, Rgenix, Sonnet, and Takeda Oncology; service on a Data and Safety Monitoring Board for Incyte Corporation, Novartis, SpringWorks, and Takeda Oncology; support for travel/accommodations and expenses from Amgen, Bristol Myers Squibb, Celgene, Clovis Oncology, Eli Lilly & Company, EMD Serono, Genentech, Merck, Mirati Therapeutics; stock and other ownership interests in Nucleai, Rgenix, and Sonnet; and stock options in Inspirina and Nucleai outside the submitted work. David P. Carbone reports honoraria from AstraZeneca and Bristol Myers Squibb‐Ono Pharmaceutical; and personal/consulting or advisory fees from AbbVie, Amgen, Arcus Biosciences, AstraZeneca, Arcus Biosciences, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Bristol Myers Squibb Israel, Curio Science, Daiichi Sankyo, Dava Oncology LP, Eli Lilly & Company, EMD Serono, F. Hoffmann‐LaRoche, G1 Therapeutics, Genentech/Roche, GlaxoSmithKline LLC, Intellisphere, InThought, Iovance Biotherapeutics, Janssen Pharmaceuticals, Jazz Pharmaceuticals, Johnson & Johnson Health Care Systems Inc., Johnson & Johnson/Janssen, Lumanity Commercial BioConsulting, Merck & Company Inc., Merck KGgA, Mirati Therapeutics, MSD Oncology, Novartis, Novocure, OncoHost, OncoLive/MJH Life Sciences, Pfizer, PPD, Regeneron Pharmaceuticals, Roche/Genentech, and Sanofi outside the submitted work. Jennifer W. Carlisle reports grants/contracts from Daiichi Sankyo, Genentech, and PAREXEL International; institutional research funding from Amgen, AstraZeneca, Hutchmed, and Parexel; and personal/consulting or advisory fees from Amgen, AstraZeneca, Catalyst Pharmaceuticals Inc., Novocure, and Sanofi outside the submitted work. Noura J. Choudhury reports institutional research funding from AbbVie, Amgen, Harpoon Therapeutics, Merck, MonteRosa Therapeutics, and Roche/Genentech; personal/consulting or advisory fees from AbbVie, Eli Lilly & Company, Harpoon Therapeutics, Sanofi, and SG1 Therapeutics; and royalties from Wolters Kluwer outside the submitted work. Jeffrey M. Clarke reports grants/contracts from Abel Zeta, AdaptImmune, Amgen, AstraZeneca, BioThera, Bristol Myers Squibb, Grid Therapeutics, Moderna, Pfizer, Spectrum Pharmaceuticals, and Zai; personal/consulting or advisory fees from AbbVie, Amgen, AstraZeneca, Black Diamond, Bristol Myers Squibb, CDR Life, Coherus BioSciences, Corbus, DSI, G1 Therapeutics, Merck, Moderna, Sanofi, and Spectrum Pharmaceuticals; service on a Data and Safety Monitoring Board for BioThera and G1 Therapeutics; and support for other professional activities from Amgen outside the submitted work. Shirish M. Gadgeel reports institutional research funding from Amgen, Astellas Pharma, AstraZeneca, BioMed Valley Discoveries, Blueprint Medicines, Calithera Biosciences, Genentech/Roche, Daiichi Sankyo, Debiopharm Group, Dragonfly Therapeutics, eFFECTOR Therapeutics, Elevation Oncology, Erasca Inc., Helsinn Therapeutics, I‐Mab, Incyte, InventisBio, Janssen Oncology, Merck, Mirati Therapeutics, Nektar, Numab, Pfizer, Regeneron, Turning Point Therapeutics, Verastem, and Ymabs Therapeutics; personal/consulting or advisory fees from AbbVie, Amgen, Arcus Biosciences, Astellas Pharma, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Eisai, Eli Lilly & Company, Genentech/Roche, Gilead Sciences, GlaxoSmithKline, I‐MAB, Janssen Oncology, Merck, Mirati Therapeutics, Novartis, Nuvation Bio, Pfizer, Regeneron, and Takeda Oncology; honoraria from Merck; support for other professional activities from AstraZeneca; and travel support from Merck and Mirati Therapeutics outside the submitted work. Hiroki Izumi reports grants/contracts from AbbVie, Amgen, AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, and Takeda; institutional research funding from AbbVie, Amgen, ArriVent Biopharma, AstraZeneca, Eisai/MSD, Ono Pharmaceutical, and Takeda; and honoraria from Bristol Myers Squibb Japan, Chugai/Roche, Eli Lilly & Company, Merck, Merck Sharp & Dohme, Ono Pharmaceutical, and Takeda outside the submitted work. Alejandro Navarro reports personal/consulting or advisory fees from Adium Pharma, Amgen, Boehringer Ingelheim, Bristol Myers Squibb Foundation, Eczacibasi, Genomics, Oryzon, Pfizer, and Takeda; service on a Data and Safety Monitoring Board for Hengenix Biotech Inc. and MedSIR; speakers bureau fees from AstraZeneca Spain and Roche; support for other professional activities from Merck Sharp & Dohme; expert witness testimony for AstraZeneca, Boehringer Ingelheim Espana S.A., Bristol Myers Squibb, F. Hoffmann‐La Roche, Pfizer, and Takeda; and travel support from F. Hoffmann‐La Roche outside the submitted work. Philip E. Lammers reports personal/consulting or advisory fees from Amgen, AstraZeneca, Daiichi Sankyo, Merck, Pfizer, Roche/Genentech, Sanofi, and Teva outside the submitted work. Shuang Huang is an employee of Amgen and owns stock in the company. Ali Hamidi is an employee of Amgen and owns stock and stock options in the company. Sujoy Mukherjee is an employee of Amgen and owns stock in the company. Taofeek K. Owonikoko reports grants/contracts from Eli Lilly & Company; institutional research funding from AbbVie, Amgen, AstraZeneca/MedImmune, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Calithera Biosciences, Corvus Pharmaceuticals, G1 Therapeutics, GlaxoSmithKline, Incyte, Loxo/Lilly, Merck, Meryx Pharmaceuticals, Novartis, Oncorus, Pfizer, Regeneron, and Roche/Genentech; personal/consulting or advisory fees from Amgen, AbbVie, AstraZeneca, Bayer, BeiGene, BerGEnBio, Boehringer Ingelheim, Bristol Myers Squibb, Cambium Oncology, Celgene, Coherus Biosciences, Daiichi Sankyo, Eisai, Eli Lilly & Company, EMD Serono, Exelixis, G1 Therapeutics, Heat Biologics, Ipsen, Janssen Biotech Inc., Jazz Pharmaceuticals Inc., MedImmune, Merck, Meryx Pharmaceuticals, Novartis, Oncocyte, PharmaMar, Puma Biotechnology, Roche/Genentech, Takeda, Triptych Health Partners, and Xcovery; service on a Data and Safety Monitoring Board for Genentech; service on an End Point Review Committee for Takeda Oncology; support for other professional activities from EMD Serono, Novartis, and Roche/Genentech; an uncompensated relationship with Reflexion Medical; has patents, royalties, and/or other intellectual property related to DR4 Modulation and its Implications in EGFR‐Target Cancer Therapy Ref: 18089 provisional (collaborative search pilot; US Patent Application No. 62/670,210; June 26, 2018; co‐inventor; institutional), Overcoming Acquired Resistance to Chemotherapy Treatments Through Suppression of STAT3 (institutional), Selective Chemotherapy Treatments and Diagnostic Methods Related Thereto (institutional), and Soluble FAS Ligand as a Biomarker of Recurrence in Thyroid Cancer (provisional; patent no. 61/727,519; inventor; institutional); support for travel/accommodations and expenses from AstraZeneca and Janssen; and owns stock and has other ownership interests in Cambium Medical Technologies, Coherus Biosciences, GenCart, and Taobob LLC outside the submitted work. The remaining authors disclosed no conflicts of interest.
Figures

References
-
- US Food and Drug Administration (FDA) . FDA grants accelerated approval to tarlatamab‐dlle for extensive stage small cell lung cancer. FDA; 2024. Accessed May 21, 2024. https://www.fda.gov/drugs/resources‐information‐approved‐drugs/fda‐grant...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

