Germline BRCA testing in Denmark following invasive breast cancer: Progress since 2000
- PMID: 39876688
- PMCID: PMC11808818
- DOI: 10.2340/1651-226X.2025.42418
Germline BRCA testing in Denmark following invasive breast cancer: Progress since 2000
Abstract
Background and purpose: Despite advancements in genetic testing and expanded eligibility criteria, underutilisation of germline testing for pathogenic variants in BRCA1 and BRCA2 (BRCA) remains evident among breast cancer (BC) patients. This observational cohort study presents real-world data on BRCA testing within the context of clinical practice challenges, including incomplete family history and under-referral.
Material and methods: From the Danish Breast Cancer Group (DBCG) clinical database, we included 65,117 females with unilateral stage I-III BC diagnosed in 2000-2017, of whom 9,125 (14%) were BRCA tested. Test results spanned from 1999 to 2021. We evaluated test rates overall and in three diagnosis periods. In logistic regression models, we examined the correlation between a BRCA test and patients' age, residency region, receptor status, and diagnosis period.
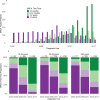
Results: Test rates rose most significantly among patients aged under 40 years, increasing from 47% (2000-2005) to 88% (2012-2017), albeit with regional discrepancies. Test timing shifted in recent years, with most results within 6 months of BC diagnosis, primarily among the youngest patients. BRCA test rates were higher for oestrogen receptor-negative/human epidermal growth factor receptor 2-negative BC (25% in 2000-2005 vs. 38% in 2012-2017), and these findings were confirmed in multivariate regression models.
Interpretation: Our results indicate a critical need for an intensified focus on BRCA testing among BC patients older than 40, where a mainstreamed testing approach might overcome delayed or missed testing. Current DBCG guidelines recommend BRCA testing of all BC patients younger than 50 years, while a general recommendation for older patients is still missing.
Conflict of interest statement
AK, AVL: Institutional and personal grants (presentation) from AstraZeneca; AVL: Advisory board MSD and economic support for congress attendance from Daiichi Sankyo (DS) and AZ; MJ: Meeting expenses and advisory board, Novartis; BE: institutional grants from AstraZeneca, Eli Lilly, MSD, Novartis, Pfizer, and Roche; meeting expenses from MSD and DS; advisory boards organised by Eli-Lilly and Medac; IP: Personal grant (presentation): AstraZeneca. KW: Personal grant (presentation), Seagen Denmark. MT, MR, and LLC report no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

