Iron-associated lipid peroxidation in Alzheimer's disease is increased in lipid rafts with decreased ferroptosis suppressors, tested by chelation in mice
- PMID: 39876821
- PMCID: PMC11775463
- DOI: 10.1002/alz.14541
Iron-associated lipid peroxidation in Alzheimer's disease is increased in lipid rafts with decreased ferroptosis suppressors, tested by chelation in mice
Abstract
Introduction: Iron-mediated cell death (ferroptosis) is a proposed mechanism of Alzheimer's disease (AD) pathology. While iron is essential for basic biological functions, its reactivity generates oxidants which contribute to cell damage and death.
Methods: To further resolve mechanisms of iron-mediated toxicity in AD, we analyzed post mortem human brain and ApoEFAD mice.
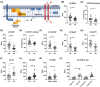
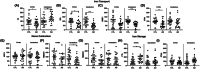
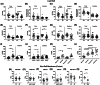
Results: AD brains had decreased antioxidant enzymes, including those mediated by glutathione (GSH). Subcellular analyses of AD brains showed greater oxidative damage and lower antioxidant enzymes in lipid rafts, the site of amyloid processing, than in the non-raft membrane fraction. Apolipoprotein E ε4 carriers had lower lipid raft yield with greater membrane oxidation. The hypothesized role of iron in AD pathology was tested in ApoEFAD mice by iron chelation with deferoxamine, which decreased fibrillar amyloid and lipid peroxidation, together with increased GSH-mediated antioxidants.
Discussion: These novel molecular pathways highlight iron-mediated damage to lipid rafts during AD.
Highlghts: Alzheimer's disease (AD) brains have numerous markers for ferroptosis, including increased lipid peroxidation, reduced antioxidant levels, and increased iron storage. Lipid rafts in AD cases have increased oxidative damage and reduced antioxidant enzyme levels and activity which are most severe in apolipoprotein E ε4 carriers. Neuronal markers are correlated with lipid peroxidation, antioxidant defense, and iron signaling proteins suggesting that neuronal loss is linked to these events. Chelation of iron in the early-onset familial AD model reduces iron-mediated lipid peroxidation and fibrillar amyloid.
Keywords: 4‐hydroxy‐nonenal; amyloid; deferoxamine; early‐onset familial Alzheimer's disease; ferritin; ferroptosis suppressor protein 1; glutathione cysteine ligase modulator; glutathione peroxidase 4.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflict of interest. Author disclosures are available in the supporting information.
Figures













Update of
-
Iron associated lipid peroxidation in Alzheimers disease is increased in lipid rafts with decreased ferroptosis suppressors, tested by chelation in mice.bioRxiv [Preprint]. 2024 Jun 13:2023.03.28.534324. doi: 10.1101/2023.03.28.534324. bioRxiv. 2024. Update in: Alzheimers Dement. 2025 Jan;21(1):e14541. doi: 10.1002/alz.14541. PMID: 37034750 Free PMC article. Updated. Preprint.
References
-
- Good PF, Perl DP, Bierer LM, Schmeidler J. Selective accumulation of aluminum and iron in the neurofibrillary tangles of Alzheimer's disease: a laser microprobe (LAMMA) study. Ann Neurol. 1992;31:286–292. - PubMed
-
- Goodman L. Alzheimer's disease; a clinico‐pathologic analysis of twenty‐three cases with a theory on pathogenesis. J Nerv Ment Dis. 1953;118:97–130. - PubMed
MeSH terms
Substances
Grants and funding
- R01-AG051521/AG/NIA NIH HHS/United States
- Glenn Foundation
- T32 AG000037/AG/NIA NIH HHS/United States
- P50-AG005142/Junior Faculty Award, and Navigage Foundation
- NIH
- P30 AG066519/AG/NIA NIH HHS/United States
- AG066530/Junior Faculty Award, and Navigage Foundation
- P30 AG066530/AG/NIA NIH HHS/United States
- NIAAG066530/ADRC
- T32AG052374/Junior Faculty Award, and Navigage Foundation
- 2022-A-010-SUP/Larry L. Hillblom Foundation
- P50 AG005142/AG/NIA NIH HHS/United States
- P50-AG05142/AG/NIA NIH HHS/United States
- RF1 AG051521/AG/NIA NIH HHS/United States
- T32 AG052374/AG/NIA NIH HHS/United States
- P01 AG055367/AG/NIA NIH HHS/United States
- P01-AG055367/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- P30AG066519/AG/NIA NIH HHS/United States
- T32-AG000037/Junior Faculty Award, and Navigage Foundation
LinkOut - more resources
Full Text Sources
Medical
Research Materials

