Second-line treatment patterns and outcomes in advanced HCC after progression on atezolizumab/bevacizumab
- PMID: 39877031
- PMCID: PMC11773230
- DOI: 10.1016/j.jhepr.2024.101232
Second-line treatment patterns and outcomes in advanced HCC after progression on atezolizumab/bevacizumab
Abstract
Background & aims: Atezolizumab/bevacizumab (A/B) is now a standard first-line treatment for advanced hepatocellular carcinoma (HCC), but the optimal second-line regimen is not known. We evaluated real-world treatment patterns and outcomes to investigate factors associated with post-progression survival (PPS).
Methods: In this multicenter, international, retrospective study, we examined clinical characteristics and outcomes of patients with advanced HCC who progressed on first-line A/B. The primary outcome of PPS was defined as time from first radiographic progression on A/B to death.
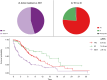
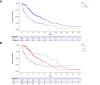
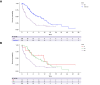
Results: A total of 406 patients alive after progression on first-line A/B were included in the final analysis, of whom 45.3% (n = 184) received best supportive treatment (BST) and 54.7% (n = 222) continued active systemic treatment. In the second line, 155 patients were treated with tyrosine kinase inhibitors (TKIs), 45 with immune checkpoint inhibitor (IO)-based regimens, and 3 had missing data. Median PPS of the whole cohort (mPPS) was 6.0 months (95% CI 5.2-7.2). On multivariate Cox regression analysis, absence of portal vein tumor thrombus, ECOG <2, and continued active treatment were predictors of better PPS. mPPS was significantly longer for patients who continued active treatment vs. BST (9.7 vs. 2.6 months; HR 0.41, p <0.001). In the second-line setting, patients treated with TKIs had a numerically shorter mPPS compared to those treated with IO (8.4 vs. 14.9 months; HR 1.37, p = 0.256).
Conclusions: Continuation of active therapy after A/B progression was independently associated with better survival even after adjusting for baseline disease characteristics. mPPS with IO-based therapy exceeded a year, suggesting that IO continuation post-progression may retain benefit. The precise sequencing of TKI and IO regimens warrants further investigation.
Impact and implications: There is currently a lack of level 1 data on second-line treatment options for patients with advanced hepatocellular carcinoma who progress after frontline atezolizumab plus bevacizumab, as all second-line approvals were established during the frontline sorafenib era. Our study aims to fill in some of the knowledge gap by investigating real-world patient outcomes in the second-line treatment setting. Findings from this study show that patients who continued active treatment had improved post-progression survival compared to those who received best supportive care, and medication regimens incorporating tyrosine kinase inhibitors as well as immunotherapy agents were active. These results can help inform clinicians of possible treatment options for patients who progress after frontline atezolizumab plus bevacizumab while we await maturing data from randomized-controlled trials.
Keywords: Hepatocellular carcinoma; atezolizumab; bevacizumab; immune checkpoint inhibitors; immunotherapy; second-line therapy; tyrosine kinase inhibitors.
© 2024 The Authors.
Conflict of interest statement
Celina Ang has received compensation for participating in advisory boards for Bayer and Caris Life Sciences. Lorenz Balcar has served as a speaker for Chiesi and Gilead. Ciro Celsa has received speaker fees or been on the advisory boards of AstraZeneca, MSD/Eisai, Ipsen and has received travel support from Roche. Hong Jae Chon holds consulting or advisory roles with Eisai, Roche, Bayer, ONO, MSD, BMS, Celgene, Sanofi, Servier, AstraZeneca, SillaJen, Menarini, and GreenCross Cell, and has received research grants from Roche, Dong-A ST, and Boryung Pharmaceuticals. Alessio Cortellini received grants for consultancies/advisory boards: BMS, MSD, OncoC4, IQVIA, Roche, GSK, AstraZeneca, Access Infinity, Ardelis Health and REGENERON. He also received speaker fees from AstraZeneca, EISAI, MSD, SANOFI/REGENERON and Pierre-Fabre. Antonio D’Alessio received educational support for congress attendance from Roche, and consultancy fees from Roche, Astrazeneca, and Chugai. Johann von Felden received honoraria from Roche and AstraZeneca. Friedrich Foerster has received honoraria for lectures from AstraZeneca, Lilly, MSD, Pfizer and Roche. He has served as advisory board member to AstraZeneca, BMS, Eisai and Roche and has received travel support from Merck KGaA and Servier. Peter Galle reports honoraria for advisory boards and lectures from Bayer, Boston Scientific, AstraZeneca, Adaptimmune, BMS, Eisai, MSD, Sirtex, Lilly, Roche, Guerbet, Ipsen. Wei-Fan Hsu received speaker fees from AstraZeneca, Eisai, and Roche. Ahmed O Kaseb received research support from Genentech, BMS, Merck, Eisai, Exelixis, AdaptImmune and Tvardi and has been on the advisory board/has received honoraria from/been a consultant for: Genentech, BMS, Merck, Eisai, Exelixis. Masatoshi Kudo has received lecture fees from Eisai and Chugai and has served as an advisor for Roche, BMS, Ono, AstraZeneca, MSD, and Eisai. Neehar Parikh has served as a consultant or advisor for Genentech, Astra Zeneca, Exact Sciences, Eisai, Fujifilm Medical, and Gilead. Matthias Pinter received speaker honoraria from Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he is a consultant/advisory board member for AstraZeneca, Bayer, BMS, Eisai, Ipsen, Lilly, MSD, and Roche; he received grants from AstraZeneca, Bayer, BMS, Eisai, and Roche; he received travel support from Bayer, BMS, Ipsen, and Roche. Tiziana Pressiani received consulting fees from Bayer, Ipsen, and AstraZeneca; institutional research funding from Roche, Bayer, Astra Zeneca; travel expenses from Roche. Lorenza Rimassa received consulting fees from AbbVie, AstraZeneca, Basilea, Bayer, Elevar Therapeutics, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Jazz Pharmaceuticals, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, Zymeworks; lecture fees from AstraZeneca, Bayer, BMS, Incyte, Ipsen, Roche, Servier; travel expenses from AstraZeneca; research grants (to Institution) from Agios, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Servier, Zymeworks. Anwaar Saeed reports a leadership role with Autem therapeutics, Exelixis, KAHR medical and Bristol-Myers Squibb; consulting or advisory board role with AstraZeneca, Bristol-Myers Squibb, Merck, Exelixis, Pfizer, Xilio therapeutics, Taiho, Amgen, Autem therapeutics, KAHR medical, and Daiichi Sankyo; institutional research funding from AstraZeneca, Bristol-Myers Squibb, Merck, Clovis, Exelixis, Actuate therapeutics, Incyte Corporation, Daiichi Sankyo, Five prime therapeutics, Amgen, Innovent biologics, Dragonfly therapeutics, Oxford Biotherapeutics, Arcus therapeutics, and KAHR medical; and participation as a data safety monitoring board chair for Arcus therapeutics. Bernhard Scheiner received grant support from AstraZeneca and Eisai, speaker honoraria from Eisai as well as travel support from AbbVie, AstraZeneca, Ipsen and Gilead. Kornelius Schulze received honoraria from MSD, Roche, and AstraZeneca. Amit Singal has served as a consultant or been on advisory boards for Genentech, AstraZeneca, Eisai, Bayer, Exelixis, Merck, Boston Scientific, Sirtex, HistoSonics, FujiFilm Medical Sciences, Exact Sciences, Glycotest, and Roche. Susanna V Ulahannan reports advisory board roles with Eisai, Astra Zeneca, and IGM biosciences. She has received institutional support for research from AbbVie, Adlai Nortye, Artios Pharma, ArQule, Inc, Astra Zeneca, Atreca, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene Corporation, Ciclomed, Eisai, Erasca, Evelo Biosciences, Exelexis, G1 Therapeutics, GlaxoSmithKline, IGM biosciences, Immunitas Therapeutics, Incyte, Isofol, Jazz Pharmaceuticals, Klus Pharma, Macrogenics, Merck, Mersana Therapeutics, Omega Therapeutics, OncoMed Pharmaceuticals, Pfizer, Purple, Regeneron, Revolution Medicines, Synermore Biologics, Takeda, Tarveda Therapeutics, Tesaro, Tempest Therapeutics, Theradex, Totos Medicines, Tvardi Therapeutics, Vigeo Therapeutics Inc. Arndt Vogel reports consultancy, speaker and advisory roles at: AstraZeneca, Amgen, BeiGene, Böhringer Mannheim, BMS, EISAI, Incyte, Ipsen, MSD, PierreFabre, Roche, Servier, and Tahio. Arndt Weinmann received compensations as a member of scientific advisory boards for AstraZeneca, Bayer, BMS, MSD, Eisai, Servier and Sanofi. He served as a speaker for Leo Pharma, Eisai, Ipsen, Abbvie, AstraZeneca and Roche and received travel support from Merck and Servier. Reports no COI: Claudia AM Fulgenzi, Joshua Glover, Yi-Hsiang Huang, Angelo Pirozzi, Giulia F Manfredi, Yehia Mohamed, Naoshi Nishida, Alessandro Parisi, Natascha Roehlen, Martin Schönlein, Marianna Silletta, Bernardo Stefanini, Y. Linda Wu, Meng Wu. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

