Histopathological characterization of skin and muscle lesions induced by lionfish (Pterois volitans) venom in a murine experimental model
- PMID: 39877151
- PMCID: PMC11773604
- DOI: 10.1590/1678-9199-JVATITD-2024-0050
Histopathological characterization of skin and muscle lesions induced by lionfish (Pterois volitans) venom in a murine experimental model
Abstract
Background: Fish venoms have been poorly characterized and the available information about their composition suggests they are uncomplicated secretions that, combined with epidermal mucus, could induce an inflammatory reaction, excruciating pain, and, in some cases, local tissue injuries.
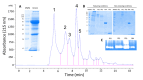
Methods: In this study, we characterized the 24-hour histopathological effects of lionfish venom in a mouse experimental model by testing the main fractions obtained by size exclusion-HPLC. By partial proteomics analysis, we also correlated these in vivo effects with the presence of some potentially toxic venom components.
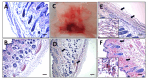
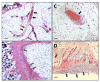
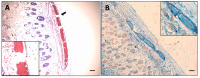
Results: We observed a strong lesion on the skin and evident necrosis in the skeletal muscle. None of the tissue-damaging effects were induced by the fraction containing cytolysins, membrane pore-forming toxins ubiquitously present in species of scorpionfish, stonefish, and lionfish, among others. On the contrary, injuries were associated with the presence of other components, which have remained practically ignored so far. This is the case of an abundant protein, present in venom, with homology to a Golgi-associated plant pathogenic protein 1-like (GAPR1), which belongs to the same protein superfamily as venom CRISPs and insect allergens.
Conclusion: This GAPR1-like protein and the hyaluronidase are probably responsible for the hemostasis impairment and hemorrhagic lesions observed in mouse skin, whereas muscle injuries can be indirectly caused by a combination of inflammatory and hemorrhagic events. More information is required to establish the components accountable for the myonecrotic effect.
Keywords: GAPR1; hyaluronidase; lionfish; myonecrosis; skin lesion; venom.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Harris RJ. The piscine arsenal: an updated review of venomous fishes. Rev Fish Biol Fisheries. 2024;34(2):539–574. doi: 10.1007/s11160-023-09828-w.. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
