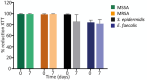Is an oritavancin catheter lock solution active against biofilms of staphylococci and enterococci?
- PMID: 39877599
- PMCID: PMC11773058
- DOI: 10.1016/j.heliyon.2025.e41885
Is an oritavancin catheter lock solution active against biofilms of staphylococci and enterococci?
Abstract
Background: Oritavancin (ORT) is a new single-dose lipoglycopeptide showing in vitro activity against staphylococci and vancomycin-resistant enterococci. However, there is no data regarding its potential use as a catheter lock solution are scarce. We constructed an in vitro model to analyze the efficacy and stability of an ORT lock solution against the biofilm of staphylococci and enterococci over 7 days at 37 °C.
Methods: We used Staphylococcus aureus, Staphylococcus epidermidis, and vancomycin-susceptible Enterococcus faecalis ATCC strains. We performed a metabolic activity assay using a 2-mg/ml solution of ORT over a 7-day incubation period at 37 °C. The solution was tested against 24-h biofilms of each strain at day 0 and 7. Metabolic activity was measured using the XTT assay, and median absorbance obtained at 490 nm in the spectrophotometer was compared between day 0 and day 7.
Results: The percentage reduction in metabolic activity was 95.3 % between biofilms treated with ORT solution incubated for 7 days and biofilms treated with ORT solution before incubation.
Conclusion: Ours is a proof-of-concept study that shows ORT to be a potential treatment as a catheter lock solution for eradication of staphylococcal and E. faecalis biofilms. It is needed to further test ORT against more clinical strains and to compare its activity with other antimicrobials in a biofilm model.
Keywords: Activity; Catheter lock solution; Catheter-related bloodstream infection; Oritavancin; Stability.
© 2025 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Maria Guembe reports financial support was provided by 10.13039/501100004587Carlos III Health Institute. Marta Diaz-Navarro reports financial support was provided by 10.13039/501100004587Carlos III Health Institute. Andres Visedo reports financial support was provided by 10.13039/100012818Community of Madrid Department of Education Science and Universities. Maria Guembe reports financial support was provided by Mutua Madrileña Foundation. Maria Guembe reports financial support was provided by Gregorio Maranon Health Research Institute. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Flüh G., Seifert H., Kaasch A.J. Oritavancin: an update. Future Microbiol. 2018;13:727–729. - PubMed
-
- Morrisette T., Miller M.A., Montague B.T., Barber G.R., McQueen R.B., Krsak M. On- and off-label utilization of dalbavancin and oritavancin for Gram-positive infections. J. Antimicrob. Chemother. 2019;74(8):2405–2416. - PubMed
-
- Mendes R.E., Castanheira M., Farrell D.J., Flamm R.K., Sader H.S., Jones R.N. Longitudinal (2001-14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010-13) analysis of oritavancin in vitro potency. J. Antimicrob. Chemother. 2016;71(12):3453–3458. - PubMed
-
- Sweeney D., Stoneburner A., Shinabarger D.L., Arhin F.F., Belley A., Moeck G., et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J. Antimicrob. Chemother. 2017;72(2):622–624. - PubMed
LinkOut - more resources
Full Text Sources