Host cell responses to Candida albicans biofilm-derived extracellular vesicles
- PMID: 39877654
- PMCID: PMC11772320
- DOI: 10.3389/fcimb.2024.1499461
Host cell responses to Candida albicans biofilm-derived extracellular vesicles
Abstract
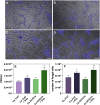
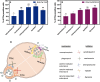
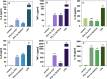
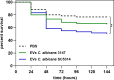
Candida albicans is a prevalent fungal pathogen responsible for infections in humans. As described recently, nanometer-sized extracellular vesicles (EVs) produced by C. albicans play a crucial role in the pathogenesis of infection by facilitating host inflammatory responses and intercellular communication. This study investigates the functional properties of EVs released by biofilms formed by two C. albicans strains-3147 (ATCC 10231) and SC5314-in eliciting host responses. We demonstrate the capability of C. albicans EVs to trigger reactions in human epithelial and immune cells. The involvement of EVs in pathogenesis was evidenced from the initial stages of infection, specifically in adherence to epithelial cells. We further established the capacity of these EVs to induce cytokine production in the epithelial A549 cell line, THP-1 macrophage-like cells, and blood-derived monocytes differentiated into macrophages. Internalization of EVs by THP-1 macrophage-like cells was confirmed, identifying macropinocytosis and phagocytosis as the most probable mechanisms, as demonstrated using various inhibitors that target potential vesicle uptake pathways in human cells. Additionally, C. albicans EVs and their cargo were identified as chemoattractants for blood-derived neutrophils. After verification of the in vivo effect of biofilm-derived EVs on the host, using Galleria mellonella larvae as an alternative model, it was demonstrated that vesicles from C. albicans SC5314 increased mortality in the injected larvae. In conclusion, for both types of EVs a predominantly pro-inflammatory effect on host was observed, highlighting their significant role in the inflammatory response during C. albicans infection.
Keywords: Candida albicans; biofilm; candidiasis; extracellular vesicles; host immune response; pathogenic fungi.
Copyright © 2025 Kulig, Wronowska, Juszczak, Zawrotniak, Karkowska-Kuleta and Rapala-Kozik.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures





Similar articles
-
Functional properties of Candida albicans extracellular vesicles released in the presence of the antifungal drugs amphotericin B, fluconazole and caspofungin.Microbiology (Reading). 2025 Jun;171(6):001565. doi: 10.1099/mic.0.001565. Microbiology (Reading). 2025. PMID: 40504166 Free PMC article.
-
Candida albicans Modifies the Protein Composition and Size Distribution of THP-1 Macrophage-Derived Extracellular Vesicles.J Proteome Res. 2017 Jan 6;16(1):87-105. doi: 10.1021/acs.jproteome.6b00605. Epub 2016 Nov 2. J Proteome Res. 2017. PMID: 27740763
-
Candida albicans biofilm extracellular vesicles deliver candidalysin to epithelial cell membranes and induce host cell responses.Infect Immun. 2025 May 13;93(5):e0040424. doi: 10.1128/iai.00404-24. Epub 2025 Apr 2. Infect Immun. 2025. PMID: 40172491 Free PMC article.
-
Candidalysin biology and activation of host cells.mBio. 2025 Jun 11;16(6):e0060324. doi: 10.1128/mbio.00603-24. Epub 2025 Apr 28. mBio. 2025. PMID: 40293285 Free PMC article. Review.
-
Macrophage pyroptosis induced by Candida albicans.Pathog Dis. 2024 Feb 7;82:ftae003. doi: 10.1093/femspd/ftae003. Pathog Dis. 2024. PMID: 38499444 Free PMC article. Review.
Cited by
-
Things you wanted to know about fungal extracellular vesicles (but were afraid to ask).PLoS Negl Trop Dis. 2025 May 22;19(5):e0013038. doi: 10.1371/journal.pntd.0013038. eCollection 2025 May. PLoS Negl Trop Dis. 2025. PMID: 40403031 Free PMC article. No abstract available.
-
Characterization and virulence of yeasts associated with neonatal thrush.BMC Microbiol. 2025 Jun 26;25(1):366. doi: 10.1186/s12866-025-04075-4. BMC Microbiol. 2025. PMID: 40571928 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

