Resonant song recognition and the evolution of acoustic communication in crickets
- PMID: 39877900
- PMCID: PMC11773217
- DOI: 10.1016/j.isci.2024.111695
Resonant song recognition and the evolution of acoustic communication in crickets
Abstract
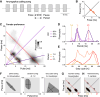
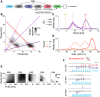
Cricket song recognition is thought to evolve through modifications of a shared neural network. However, the species Anurogryllus muticus has an unusual recognition pattern that challenges this view: females respond to both normal male song pulse periods and periods twice as long. Of the three minimal models tested, only a single-neuron model with an oscillating membrane could explain this unusual behavior. A minimal model of the cricket's song network reproduced the behavior after adding a mechanism that, while present in the full network, is not crucial for song recognition in other species. This shows how a shared neural network can produce diverse behaviors and highlights how different computations contribute to evolution. Our results also demonstrate how nonlinear computations can lead to rapid behavioral changes during evolution because small changes in network parameters can lead to large changes in behavior.
Keywords: Bioacoustics; Entomology; Neuroscience.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
A small, computationally flexible network produces the phenotypic diversity of song recognition in crickets.Elife. 2021 Nov 11;10:e61475. doi: 10.7554/eLife.61475. Elife. 2021. PMID: 34761750 Free PMC article.
-
Time and timing in the acoustic recognition system of crickets.Front Physiol. 2014 Aug 12;5:286. doi: 10.3389/fphys.2014.00286. eCollection 2014. Front Physiol. 2014. PMID: 25161622 Free PMC article.
-
Singing crickets from Brazil (Orthoptera: Gryllidea), an illustrated checklist with access to the sounds produced.Zootaxa. 2022 Nov 16;5209(2):211-237. doi: 10.11646/zootaxa.5209.2.4. Zootaxa. 2022. PMID: 37045396
-
Sequential Filtering Processes Shape Feature Detection in Crickets: A Framework for Song Pattern Recognition.Front Physiol. 2016 Feb 25;7:46. doi: 10.3389/fphys.2016.00046. eCollection 2016. Front Physiol. 2016. PMID: 26941647 Free PMC article. Review.
-
Neuroethology of acoustic communication in field crickets - from signal generation to song recognition in an insect brain.Prog Neurobiol. 2020 Nov;194:101882. doi: 10.1016/j.pneurobio.2020.101882. Epub 2020 Jul 13. Prog Neurobiol. 2020. PMID: 32673695 Review.
References
LinkOut - more resources
Full Text Sources

