Transient Triplet Metallopnictinidenes M-Pn (M = PdII, PtII; Pn = P, As, Sb): Characterization and Dimerization
- PMID: 39878059
- PMCID: PMC11826990
- DOI: 10.1021/jacs.4c16830
Transient Triplet Metallopnictinidenes M-Pn (M = PdII, PtII; Pn = P, As, Sb): Characterization and Dimerization
Abstract
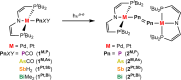
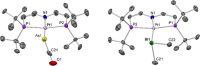
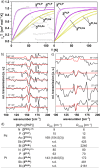
Nitrenes (R-N) have been subject to a large body of experimental and theoretical studies. The fundamental reactivity of this important class of transient intermediates has been attributed to their electronic structures, particularly the accessibility of triplet vs singlet states. In contrast, electronic structure trends along the heavier pnictinidene analogues (R-Pn; Pn = P-Bi) are much less systematically explored. We here report the synthesis of a series of metallodipnictenes, {M-Pn═Pn-M} (M = PdII, PtII; Pn = P, As, Sb, Bi) and the characterization of the transient metallopnictinidene intermediates, {M-Pn} for Pn = P, As, Sb. Structural, spectroscopic, and computational analysis revealed spin triplet ground states for the metallopnictinidenes with characteristic electronic structure trends along the series. In comparison to the nitrene, the heavier pnictinidenes exhibit lower-lying ground state SOMOs and singlet excited states, thus suggesting increased electrophilic reactivity. Furthermore, the splitting of the triplet magnetic microstates is beyond the phosphinidenes {M-P} dominated by heavy pnictogen atom induced spin-orbit coupling.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Falvey D. E.; Gudmundsdottir A. D.. Nitrenes and Nitrenium Ions, 1st ed.; J. Wiley & Sons, 2013.
- Albini A.; Fagnioni M.. Photogeneration of Carbenes and Nitrenes. In Photochemically-Generated Intermediates in Synthesis; J. Wiley & Sons, 2013; pp 302–327.
-
- Gritsan N. P.; Platz M. S. Kinetics, Spectroscopy, and Computational Chemistry of Arylnitrenes. Chem. Rev. 2006, 106, 3844–3867. 10.1021/cr040055+. - DOI - PubMed
- Wentrup C. Flash Vacuum Pyrolysis of Azides, Triazoles, and Tetrazoles. Chem. Rev. 2017, 117, 4562–4623. 10.1021/acs.chemrev.6b00738. - DOI - PubMed
- Wentrup C. Carbenes and Nitrenes: Recent Developments in Fundamental Chemistry. Angew. Chem., Int. Ed. 2018, 57, 11508–11521. 10.1002/anie.201804863. - DOI - PubMed
-
- Salem L.; Rowland C. The Electronic Properties of Diradicals. Angew. Chem. Int. Ed. 1972, 11, 92–111. 10.1002/anie.197200921. - DOI
- Bonačić-Koutecký V.; Koutecký J.; Michl J. Neutral and Charged Biradicals, Zwitterions, Funnels in S1, and Proton Translocation: Their Role in Photochemistry, Photophysics, and Vision. Angew. Chem., Int. Ed. 1987, 26, 170–189. 10.1002/anie.198701701. - DOI
-
- Huo W. M. Valence Excited States of NH and CH and Theoretical Transition Probabilities. J. Chem. Phys. 1968, 49, 1482–1492. 10.1063/1.1670269. - DOI
- O’Neil S. V.; Schaefer H. F. Configuration Interaction Study of the X3Σ–, a1Δ, and b1Σ+ States of NH. J. Chem. Phys. 1971, 55, 394–401. 10.1063/1.1675534. - DOI
-
- Yarkony D. R.; Schaefer I. I. I.; Rothenburg S. X. 3A2, a 1E, and b 1A1 Electronic States of Methylnitrene. J. Am. Chem. Soc. 1974, 96, 5974–5977. 10.1021/ja00826a003. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

