VopX, a novel Vibrio cholerae T3SS effector, modulates host actin dynamics
- PMID: 39878476
- PMCID: PMC11898728
- DOI: 10.1128/mbio.03018-24
VopX, a novel Vibrio cholerae T3SS effector, modulates host actin dynamics
Abstract
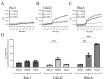
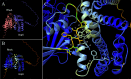
Pathogenic Vibrio cholerae strains cause cholera using different mechanisms. O1 and O139 serogroup strains use the toxin-co-regulated pilus (TCP) and cholera toxin (CT) for intestinal colonization and to promote secretory diarrhea, while non-O1/non-O139 serogroup strains are typically non-toxigenic and use alternate virulence factors to cause a clinically similar disease. An O39 serogroup, TCP/CT-negative V. cholerae strain, named AM-19226, uses a type III secretion system (T3SS) to translocate more than 10 effector proteins into the host cell cytosol. Effectors VopF and VopM directly interact with the host actin and contribute to colonization. Our previous studies using the Saccharomyces cerevisiae model system identified VopX as a third effector that alters cytoskeletal dynamics. Herein, we used complementary approaches to translate yeast findings to a mammalian system and determined the target and mechanism of VopX activity. VopX overexpression in HeLa cells caused dramatic cell rounding. Co-culture of strain AM-19226 with polarized Caco-2/BBE monolayers increased formation of stress fibers and focal adhesions, as well as Caco-2/BBE adherence to extracellular matrix in a VopX-dependent manner. Finally, we demonstrate in vitro that VopX can act as a guanine nucleotide exchange factor for RhoA, which functions upstream of a mitogen-activated protein kinase (MAPK) signaling pathway regulating cytoskeletal dynamics. Our results suggest that VopX activity initiates a signaling cascade resulting in enhanced cell-extracellular matrix adhesion, potentially preventing detachment of host cells, and facilitating sustained bacterial colonization during infection. VopX function is therefore part of a unique pathogenic strategy employed by T3SS-positive V. cholerae, which involves multiple cytoskeletal remodeling mechanisms to support a productive infection.
Importance: Despite different infection strategies, enteric pathogens commonly employ a T3SS to colonize the human host and cause disease. Effector proteins are unique to each T3SS-encoding bacterial species and generally lack conserved amino acid sequences. However, T3SS effectors from diverse pathogens target and manipulate common host cell structures and signaling proteins, such as the actin cytoskeleton and MAPK pathway components. T3SS-encoding Vibrio cholerae strains and effectors have been relatively recently identified, and the mechanisms used to mediate colonization and secretory diarrhea are poorly understood. Two V. cholerae effectors that modify the host actin cytoskeleton were shown to be important for colonization. We therefore sought to determine the target(s) and mechanism of a third actin-reorganizing effector, VopX, based on results obtained from a yeast model system. We recapitulated actin-based phenotypes in multiple mammalian model systems, leading us to identify the molecular function of the V. cholerae VopX effector protein.
Keywords: T3SS; V. cholerae; cytoskeleton; effector; pathogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
- R01 AI073785/AI/NIAID NIH HHS/United States
- R01 AI126005/AI/NIAID NIH HHS/United States
- R01 DK131550/DK/NIDDK NIH HHS/United States
- R01AI126005, R01AI073785/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01DK131550/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
LinkOut - more resources
Full Text Sources
