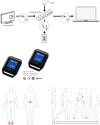
Comparison of in-clinic assessment of 6MWT by conventional method and using wearable sensors for patients with ATTR-CM
- PMID: 39878480
- PMCID: PMC11812369
- DOI: 10.1080/14796678.2025.2457881
Comparison of in-clinic assessment of 6MWT by conventional method and using wearable sensors for patients with ATTR-CM
Abstract
Introduction: The 6-minute walk test (6MWT) is used to assess submaximal exercise capacity in clinical trials. Conducting the 6MWT can be challenging when patients cannot visit the clinic due to physical/travel limitations. This pilot study assessed the feasibility of conducting the 6MWT using wearable sensors for patients with transthyretin amyloid cardiomyopathy.
Methods: Participants were enrolled in the phase 3 ATTRibute-CM trial. Sensors were positioned on patients' feet and lower back during the 6MWT. The 6-minute walk distance (6MWD) was compared with the distance measured by a trained observer during a concurrent conventional test. Pearson and concordance correlation coefficients were estimated.
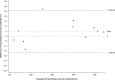
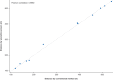
Results: Twelve participants from five centers participated; 11 had evaluable data. Mean 6MWD was 330.3 m (conventional method) and 335.1 m (wearable sensors); mean difference (SD) was 4.7 m (10.95). Pearson and concordance correlation coefficients for 6MWD were 0.998 (95% CI: 0.992-0.999) and 0.997 (95% CI: 0.991-0.999), respectively.
Conclusions: The 6MWD measured using wearable sensors and by the conventional method were closely correlated. Conducting the 6MWT with wearable sensors may be feasible and as reliable as the conventional method in a monitored clinic setting. Whether at-home 6MWD measured by wearable sensors correlates with in-clinic monitoring deserves further study.
Clinical trial registration: ClinicalTrials.gov identifier is NCT03860935.
Keywords: 6-minute walk test; 6MWD; ATTR-CM; exercise capacity; heart failure; transthyretin amyloidosis; wearable sensors.
Plain language summary
The 6-minute walk test (6MWT) assesses the ability of a person to do physical exercise by measuring the distance walked in 6 minutes. It is commonly used in clinical trials involving patients with heart disease. The recent COVID-19 pandemic exposed the potential for clinical trials to be disrupted by unforeseen circumstances. During the pandemic, patients were unable to travel to hospitals or physician offices to take part in clinical trial visits. In this study, scientists tested whether wearable sensors can be used to measure the distance walked during a 6MWT with the idea that this technology could eventually be used to remotely monitor a patient doing the 6MWT at home. The study included 12 patients with transthyretin amyloid cardiomyopathy, a condition where a protein called transthyretin builds up in the heart muscle and disrupts its normal function, leading to heart failure. Wearable sensors were put on the feet and lower back of the patients during the 6MWT. The distance the patient walked when wearing the sensor was compared with the distance walked during the normal 6MWT. The results showed that the distances measured by the sensors and by the usual test were very similar. This means that it might be possible to use wearable sensors for the 6MWT, and that there may be a potential to carry out the test without the need for in-person clinic visits. Additional studies in more patients and in real-life settings are required to confirm these results.
Conflict of interest statement
Prem Soman has received grants from Pfizer and served on consulting/advisory boards for Alnylam Pharmaceuticals, BridgeBio Pharma, Inc., Pfizer, and Spectrum Dynamics. Michel G. Khouri has received institutional research support from Alnylam Pharmaceuticals, BridgeBio Pharma, Inc., Ionis Pharmaceuticals, and Pfizer; has received consulting/advisory board support from Alnylam Pharmaceuticals and BridgeBio Pharma, Inc.; and is a member of the Alnylam Pharmaceuticals speakers bureau. Daniel Lenihan has participated on a data safety monitoring board for Intellia and has been a consultant for Myocardial Solutions, Inc. Alex Reyentovich declares nothing to report. Brett W. Sperry has been a speaker for Pfizer and a consultant for Alnylam Pharmaceuticals, AstraZeneca, and BridgeBio Pharma, Inc. Kristen Sowalsky is an employee of Clario and may own company stock. Yun Bai, Jing Du, Leonid Katz, Suresh Siddhanti, and Jonathan C. Fox are employees and shareholders of BridgeBio Pharma, Inc. Editorial assistance was provided by Eleanor Foy, PhD, of PharmaGenesis London, funded by BridgeBio Pharma, Inc.
Figures
References
-
- Farwati M, Riaz H, Tang WHW.. Digital health applications in heart failure: a critical appraisal of literature. Curr Treat Options Cardiovasc Med. 2021;23:12. doi: 10.1007/s11936-020-00885-z - DOI - PMC - PubMed
-
•• A literature review that highlights the importance of digital health technologies as potentially useful tools for improving patient care and research in heart failure.
-
- Giannitsi S, Bougiakli M, Bechlioulis A, et al. 6-minute walking test: a useful tool in the management of heart failure patients. Ther Adv Cardiovasc Dis. 2019;13:1753944719870084. doi: 10.1177/1753944719870084 - DOI - PMC - PubMed
-
•• A review summarizing the value of the 6MWT in patients with heart failure, including usefulness and limitations.
-
- Imberti JF, Tosetti A, Mei DA, et al. Remote monitoring and telemedicine in heart failure: implementation and benefits. Curr Cardiol Rep. 2021;23:55. doi: 10.1007/s11886-021-01487-2 - DOI - PMC - PubMed
-
•• A review highlighting potential benefits and barriers to the implementation of remote monitoring in patients with heart failure.
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials