Management of nausea and vomiting induced by antibody-drug conjugates
- PMID: 39878905
- PMCID: PMC11842424
- DOI: 10.1007/s12282-025-01670-1
Management of nausea and vomiting induced by antibody-drug conjugates
Abstract
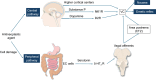
Antibody-drug conjugates (ADCs) are an emerging class of anticancer therapy that combines the specificity and long circulation half-life of monoclonal antibodies with the cytotoxic potency of the payload connected through a chemical linker. The optimal management of toxicities is crucial for improving quality of life in patients undergoing ADCs and for avoiding improper dose reductions or discontinuations. This article focuses on the characteristics and management of nausea and vomiting (NV) induced by three ADCs: trastuzumab deruxtecan (T-DXd), sacituzumab govitecan (SG), and datopotamab deruxtecan (Dato-DXd). We summarize the proposed mechanism of NV, clinical study data on NV, and recommendations from clinical guidelines. We also discuss three prospective studies evaluating prophylactic antiemetic therapy in patients receiving T-DXd, along with future perspectives.
Keywords: Antibody–drug conjugate; Datopotamab deruxtecan; Nausea and vomiting; Sacituzumab govitecan; Trastuzumab deruxtecan.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: HS has received support for the present manuscript (to institution) and medical writing support from Daiichi Sankyo, lecture fees for Daiichi Sankyo, Chugai Pharmaceutical, and Eli Lilly, advisory role fee from Gilead Sciences. JT has received support for the present manuscript from Daiichi Sankyo (to institution), research grants from Eli Lilly, Daiichi Sankyo, and AstraZeneca (to institution), personal fees for Consulting/honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events/advisory board from Daiichi Sankyo and AstraZeneca.
Figures
References
-
- Tarantino P, Ricciuti B, Pradhan SM, Tolaney SM. Optimizing the safety of antibody-drug conjugates for patients with solid tumours. Nat Rev Clin Oncol. 2023;20:558–76. - PubMed
-
- Cortés J, Kim S-B, Chung W-P, Im S-A, Park YH, Hegg R, et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N Engl J Med. 2022;386:1143–54. - PubMed
-
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529–41. - PubMed
-
- Bardia A, Jhaveri K, Im SA, Pernas S, De Laurentiis M, Wang S, et al. Datopotamab deruxtecan versus chemotherapy in previously treated inoperable/metastatic hormone receptor-positive human epidermal growth factor receptor 2-negative breast cancer: primary results from TROPION-Breast01. J Clin Oncol. 2024. 10.1200/JCO.24.00920. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

