How Do the Valency and Radii of Cations Affect the Rheological Properties of Aqueous Solutions of Zwitterionic and Anionic Surfactant Mixtures?
- PMID: 39879074
- PMCID: PMC11823622
- DOI: 10.1021/acs.langmuir.4c04689
How Do the Valency and Radii of Cations Affect the Rheological Properties of Aqueous Solutions of Zwitterionic and Anionic Surfactant Mixtures?
Abstract
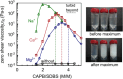
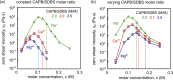
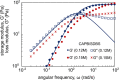
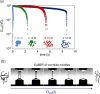
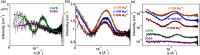
Despite extensive research on the use of salts to enhance micellar growth, numerous questions remain regarding the impact of ionic exchange and molecular structure on charge neutralization. This study looks into how certain cations (Na+, Ca2+, and Mg2+) affect the structure of a cocamidopropyl betaine CAPB and sodium dodecylbenzenesulfonate SDBS surfactant mixture, aiming toward applications in targeted delivery systems. The mixture consists of a zwitterionic surfactant, cocamidopropyl betaine (CAPB), and an anionic surfactant, sodium dodecylbenzenesulfonate (SDBS), combined in varying molar ratios at a total concentration of 200 mM. We characterized the macroscale properties through rheological measurements and obtained detailed structural insights using small-angle X-ray scattering (SAXS) and cryogenic electron microscopy (cryo-EM). The findings reveal that increasing the concentration of cations in the CAPB/SDBS mixture induces the formation of peaks in the zero-shear viscosity as a function of salt concentration (salt curve). Analysis through cryo-EM and SAXS showed that these viscosity peaks are related to the change of micellar assemblies from entangled worm-like micelles to branched worm-like micelles and then to bilayer structures (vesicles). The specific cation concentration at which the zero-shear viscosity peak occurs, as well as the maximum viscosity, is strongly influenced by the type of cation present in the CAPB/SDBS solutions, a phenomenon explained by the Hofmeister series. Notably, the differing affinities of cations for the carboxylate COO- and sulfite SO3- groups and the partial dehydration of micelles contribute to the lower concentration of magnesium cations required to reach the viscosity peak compared to calcium cations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Shaban S. M.; Kang J.; Kim D. H. Surfactants: recent advances and their applications. Compos. Commun. 2020, 22, 100537 10.1016/j.coco.2020.100537. - DOI
-
- Farias C. B. B.; Almeida F. C.; Silva I. A.; Souza T. C.; Meira H. M.; Rita de Cássia F.; Sarubbo L. A.; et al. Production of green surfactants: Market prospects. Electron. J. Biotechnol. 2021, 51, 28–39. 10.1016/j.ejbt.2021.02.002. - DOI
-
- Berret J. F. Rheology of Wormlike Micelles: Equilibrium Properties and Shear Banding Transitions. Mol. Gels 2006, 19, 667–720. 10.1007/1-4020-3689-2_20. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

