Metabolically stable apelin analogs: development and functional role in water balance and cardiovascular function
- PMID: 39879076
- PMCID: PMC12204018
- DOI: 10.1042/CS20240955
Metabolically stable apelin analogs: development and functional role in water balance and cardiovascular function
Abstract
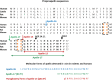
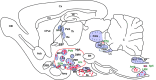
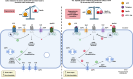
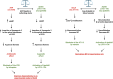
Apelin, a (neuro) vasoactive peptide, plays a prominent role in controlling water balance and cardiovascular functions. Apelin and its receptor co-localize with vasopressin in magnocellular vasopressinergic neurons. Apelin receptors (Apelin-Rs) are also expressed in the collecting ducts of the kidney, where vasopressin type 2 receptors are also present. Apelin and vasopressin interact at the brain and renal levels to maintain body fluid homeostasis by regulating diuresis in opposite directions. Apelin and angiotensin II have opposite effects on the regulation of blood pressure (BP). Angiotensin II, by binding to AT1 receptors present in VSMCs, induces intracellular calcium mobilization and vasoconstriction, while apelin, by binding to Apelin-R present on vascular endothelium, increases nitric oxide production and induces vasodilation. Apelin also plays a crucial role in the regulation of cardiac function. Apelin-deficient and Apelin-R-deficient mice develop progressive myocardial dysfunction with ageing and are susceptible to heart failure in response to pressure overload. Since the half-life of apelin is very short in vivo (in the minute range), several metabolically stable apelin analogs and non-peptidic Apelin-R agonists have been developed, with potential applications in diverse diseases. In this review, we highlight the interaction between apelin and vasopressin in the regulation of water balance and that between apelin and angiotensin II in the regulation of BP. Additionally, we underline the protective effects of apelin in cardiac function. Lastly, we discuss the beneficial effects of Apelin-R activation in different pathological states such as hyponatremia, hypertension, and heart failure.
Keywords: Apelin receptor; Aqueous diuresis; Blood pressure; Cardiac function;; G Protein-Coupled Receptor.
© 2025 The Author(s); published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

