A simple tool to evaluate the effectiveness of HIV care for settings with gaps in data availability (ESTIHIV)
- PMID: 39879174
- PMCID: PMC11778770
- DOI: 10.1371/journal.pone.0316794
A simple tool to evaluate the effectiveness of HIV care for settings with gaps in data availability (ESTIHIV)
Abstract
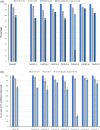
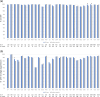
Many HIV clinics with poor IT-infrastructure are unable to report data on individuals in care with HIV, on antiretroviral treatment (ART) and virologically suppressed (VS), with the aim of monitoring the HIV Continuum of Care to estimate efficacy of HIV treatment programmes. We developed an estimation-tool, ESTIHIV, and determined the minimal data required for a random sample, to produce representative estimates, with a specified level of precision, of people with HIV on ART and VS. For proof of concept, 8852 HIV positive persons from seven clinics in seven different countries, with a follow-up visit during 2017, were included. Of those, 93.8% were on ART (95% CI 93.3-94.2) and 76.7% were VS (95% CI 75.8-77.6). In 2022, we tested the tool in the RESPOND Cohort for all countries with more than 100 participants under follow-up in 2019. We included 26,426 HIV positive persons from clinics in 27 countries, 97.8% (95% CI 97.6-98.0) were on ART and 91.5% were VS (95% CI 91.2-91.8%). There was good agreement between the RESPOND country estimates of ART and VS and the estimations using a random sample calculated in ESTIHIV. With ESTIHIV, clinics can produce a reliable estimate in figures for reporting and for monitoring the effectiveness of care in their clinics.
Copyright: © 2025 Raben et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
AM has received personal honoraria, travel support, and lecture fees from Gilead and ViiV, and consultancy fees from Eiland and Bonnin, all outside the submitted work. JG is an employee at Gilead Sciences. HG is an employee at ViiV Healthcare. For the remaining authors none were declared.
Figures
References
-
- Vourli G, Noori T, Pharris A, Porter K, Axelsson M, Begovac J, et al.. Human immunodeficiency virus continuum of care in 11 European union countries at the end of 2016 overall and by key population: have we made progress? Clin Infect Dis. 2020;71(11):2905–16. doi: 10.1093/cid/ciaa696 - DOI - PMC - PubMed
-
- HIV+ Continuum of care. Monitoring implementation of the Dublin Declaration on partnership to fight HIV/AIDS in Europe and Central Asia: 2021 progress report. Eur Centre Dis Prev Control. 2022.
-
- HIV/AIDS surveillance in Europe 2017 – 2016 data. European Centre for Disease Prevention and Control, ECDC/ WHO Regional Office for Europe; 2017.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

