European ILD registry algorithm for self-assessment in interstitial lung diseases (eurILDreg ASA-ILD)
- PMID: 39879227
- PMCID: PMC11778754
- DOI: 10.1371/journal.pone.0316484
European ILD registry algorithm for self-assessment in interstitial lung diseases (eurILDreg ASA-ILD)
Abstract
Background and aims: Predicting progression and prognosis in Interstitial Lung Diseases (ILD), especially Idiopathic Pulmonary Fibrosis (IPF) and Progressive Pulmonary Fibrosis (PPF), remains a challenge. Integrating patient-centered measurements is essential for earlier and safer detection of disease progression. Home monitoring through e-health technologies, such as spirometry and oximetry connected to smartphone applications, holds promise for early detection of ILD progression or acute exacerbations, enabling timely therapeutic interventions.
Methods: The European ILD Registry Algorithm for Self-Assessment in ILD (eurILDreg ASA-ILD), developed by all eurILDreg principal investigators, includes questionnaires on symptom burden, respiratory infections, and quality of life (EQ5D VAS, K-BILD, LCQ). The algorithm also incorporates spirometry and oxygen saturation measurements, both at rest and during exercise (one-minute sit-to-stand test, 1STST). This ASA-ILD algorithm is integrated into the patientMpower Ltd. smartphone application, used for patient-led monitoring, research, and clinical care since 2016, and available on both Apple and Android platforms.
Discussion: For patient-centered measurements, participants in the multicenter eurILDreg study will receive a patientMpower account, a handheld clinical-grade spirometer (Spirobank Smart, MIR, Italy), and a pulse oximeter (Nonin Medical, Inc. Plymouth, MN, USA), along with usage instructions. Artificial intelligence software (ArtiQ) will analyze spirometry maneuvers in real-time, ensuring compliance with recent ERS/ATS criteria and providing automated feedback. Pulse oximetry is integrated into the exercise testing within the application, following an automated in-app protocol developed with clinician involvement for safety and accuracy. The application will send reminders to participants to complete patient-reported outcome measures (PROMs) according to the study protocol.
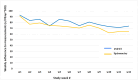
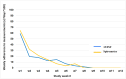
Conclusion: This study is designed to explore the potential of e-Health technologies, such as home monitoring via spirometry and oximetry, integrated with the eurILDreg ASA-ILD algorithm and patientMpower app, to improve early detection and management of ILD. A pilot trial showed promising adherence to spirometry, indicating that digital health interventions could enhance patient care and outcomes in ILD.
Trial registration: The ethics committee of the Justus-Liebig-University of Giessen has approved the eurILDreg and this substudy with the protocol reference number 111/08. The research was conducted strictly according to the principles of the Declaration of Helsinki. Patients were included into the registry upon having signed the informed consent. The eurIPFreg and eurIPFbank are listed in ClinicalTrials.gov (NCT02951416). EurILDreg is registered in German Clinical Trials Register, DRKS 00028968.
Copyright: © 2025 Krauss et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
RB is an employee of patientMpower Ltd. This does not alter our adherence to PLOS ONE policies on sharing data and materials. Other authors have no competing interests.
Figures

References
-
- Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, et al.. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–47. doi: 10.1164/rccm.202202-0399ST - DOI - PMC - PubMed
-
- Travis WD, Costabel U, Hansell DM, King TE Jr, Lynch DA, Nicholson AG, et al.. An official American thoracic society/European respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. doi: 10.1164/rccm.201308-1483ST - DOI - PMC - PubMed

