Near-infrared spectroscopy discriminates mass-reared sterile and wild tsetse flies
- PMID: 39879251
- PMCID: PMC11809883
- DOI: 10.1371/journal.pntd.0012857
Near-infrared spectroscopy discriminates mass-reared sterile and wild tsetse flies
Abstract
Background: Monitoring the efficacy of the sterile insect technique (SIT) programs, it is desirable to discriminate between wild and sterile tsetse males captured in monitoring traps. Currently, this is primarily achieved by marking sterile males with fluorescent dye powder before release, and identifying them using a fluorescence camera and/or microscope. However, the accuracy of this method is limited due to defective marking and wild flies contaminated with a few dye particles in the monitoring traps. Molecular techniques have been developed to discriminate doubtful flies, but they are expensive for endemic countries.
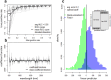
Methodology/principal findings: Here, we investigate the ability of a new generation monitoring tool, Near-Infrared Spectroscopy (NIRS), to discriminate between laboratory-reared Glossina palpalis gambiensis males and their field counterparts. NIRS was able to discriminate wild males from laboratory-reared males with 86% accuracy. Notably, the prediction accuracy improved to 88% when the laboratory-reared flies had been irradiated.
Conclusions/significance: These findings suggest that NIRS can successfully identify tsetse flies even when UV camera identification is inconclusive. However, further studies are needed to expand the training dataset and include additional environmental variables before validating NIRS as a complementary method for future tsetse eradication programs.
Copyright: © 2025 Pagabeleguem et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
A Molecular Method to Discriminate between Mass-Reared Sterile and Wild Tsetse Flies during Eradication Programmes That Have a Sterile Insect Technique Component.PLoS Negl Trop Dis. 2016 Feb 22;10(2):e0004491. doi: 10.1371/journal.pntd.0004491. eCollection 2016 Feb. PLoS Negl Trop Dis. 2016. PMID: 26901049 Free PMC article.
-
Boosting the sterile insect technique with pyriproxyfen increases tsetse flies Glossina palpalis gambiensis sterilization in controlled conditions.Sci Rep. 2020 Jun 19;10(1):9947. doi: 10.1038/s41598-020-66850-9. Sci Rep. 2020. PMID: 32561776 Free PMC article.
-
Intrinsic and synthetic stable isotope marking of tsetse flies.J Insect Sci. 2011;11:79. doi: 10.1673/031.011.7901. J Insect Sci. 2011. PMID: 21870965 Free PMC article.
-
Principles of area-wide integrated tsetse fly control using the sterile insect technique.Med Trop (Mars). 2001;61(4-5):397-411. Med Trop (Mars). 2001. PMID: 11803833 Review.
-
Tsetse salivary gland hypertrophy virus: hope or hindrance for tsetse control?PLoS Negl Trop Dis. 2011 Aug;5(8):e1220. doi: 10.1371/journal.pntd.0001220. Epub 2011 Aug 30. PLoS Negl Trop Dis. 2011. PMID: 21912708 Free PMC article. Review.
References
-
- Hursey BS, Slingenbergh J. The tsetse fly and its effects on agriculture in sub-Saharan Africa. World Animal Review. 1995; 67–73. Available from: doi: 10.1007/1-4020-4051-2_22 - DOI
-
- Swallow B. Impacts of Trypanosomosis on African Agriculture. Rome, Italy; 1999. Dec p. 46.
-
- Hendrichs J, Vreysen MJB, Enkerlin WR, Cayol JP. Strategic options in using sterile insects for area-wide integrated pest management. Sterile insect technique. In: Dyck VA, Hendrichs J, Robinson AS, editors. Sterile insect technique: Principles and practice in area-wide integrated pest management. CRC Press; 2021. pp. 841–884.
-
- Bloem S, Carpenter J, Mccluskey A, Fugger R, Arthur S, Wood S. Suppression of the Codling Moth Cydia pomonella in British Columbia, Canada Using an Area-Wide Integrated Approach with an SIT Components. In: Vreysen MJB, Robinson AS, Hendrichs J, editors. Area-Wide Control of Insect Pests. Dordrecht: Springer Netherlands; 2007. pp. 591–601. doi: 10.1007/978-1-4020-6059-5_55 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

