Replication-incompetent VSV-based vaccine elicits protective responses against SARS-CoV-2 and influenza virus
- PMID: 39879304
- PMCID: PMC11777205
- DOI: 10.1126/sciadv.adq4545
Replication-incompetent VSV-based vaccine elicits protective responses against SARS-CoV-2 and influenza virus
Abstract
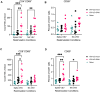
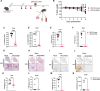
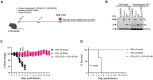
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and influenza viruses lead to severe respiratory illnesses and death in humans, exacerbated in individuals with underlying health conditions, remaining substantial global public health concerns. Here, we developed a bivalent replication-incompetent single-cycle pseudotyped vesicular stomatitis virus vaccine that incorporates both a prefusion-stabilized SARS-CoV-2 spike protein lacking a furin cleavage site and a full-length influenza A virus neuraminidase protein. Vaccination of K18-hACE2 or C57BL/6J mouse models generated durable levels of neutralizing antibodies, T cell responses, and protection from morbidity and mortality upon challenge with either virus. Furthermore, the vaccine provided heterologous protection upon challenge with a different influenza virus strain, supporting the advantage of using NA to increase the breadth of vaccine protection. Now, no bivalent vaccine is approved for use against both SARS-CoV-2 and influenza virus. Our study supports using this platform to develop safe and efficient vaccines against multiple viruses.
Figures




Similar articles
-
A decavalent composite mRNA vaccine against both influenza and COVID-19.mBio. 2024 Sep 11;15(9):e0066824. doi: 10.1128/mbio.00668-24. Epub 2024 Aug 6. mBio. 2024. PMID: 39105586 Free PMC article.
-
A chimeric mRNA vaccine of S-RBD with HA conferring broad protection against influenza and COVID-19 variants.PLoS Pathog. 2024 Sep 20;20(9):e1012508. doi: 10.1371/journal.ppat.1012508. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39303003 Free PMC article.
-
MCMV-based vaccine vectors expressing full-length viral proteins provide long-term humoral immune protection upon a single-shot vaccination.Cell Mol Immunol. 2022 Feb;19(2):234-244. doi: 10.1038/s41423-021-00814-5. Epub 2022 Jan 7. Cell Mol Immunol. 2022. PMID: 34992275 Free PMC article.
-
A Recombinant VSV-Based Bivalent Vaccine Effectively Protects against Both SARS-CoV-2 and Influenza A Virus Infection.J Virol. 2022 Sep 28;96(18):e0133722. doi: 10.1128/jvi.01337-22. Epub 2022 Sep 7. J Virol. 2022. PMID: 36069551 Free PMC article.
-
A Methyltransferase-Defective Vesicular Stomatitis Virus-Based SARS-CoV-2 Vaccine Candidate Provides Complete Protection against SARS-CoV-2 Infection in Hamsters.J Virol. 2021 Sep 27;95(20):e0059221. doi: 10.1128/JVI.00592-21. Epub 2021 Aug 11. J Virol. 2021. PMID: 34379509 Free PMC article.
References
-
- Lamers M. M., Haagmans B. L., SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 20, 270–284 (2022). - PubMed
-
- Regan J. J., Moulia D. L., Link-Gelles R., Godfrey M., Mak J., Najdowski M., Rosenblum H. G., Shah M. M., Twentyman E., Meyer S., Peacock G., Thornburg N., Havers F. P., Saydah S., Brooks O., Talbot H. K., Lee G. M., Bell B. P., Mahon B. E., Daley M. F., Fleming-Dutra K. E., Wallace M., Use of updated COVID-19 vaccines 2023–2024 formula for persons aged ≥6 months: Recommendations of the Advisory Committee on Immunization Practices—United States, September 2023. MMWR Morb. Mortal. Wkly Rep. 72, 1140–1146 (2023). - PMC - PubMed
-
- Yamayoshi S., Kawaoka Y., Current and future influenza vaccines. Nat. Med. 25, 212–220 (2019). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

