Plasma phosphorylated tau217 strongly associates with memory deficits in the Alzheimer's disease spectrum
- PMID: 39879633
- PMCID: PMC12378614
- DOI: 10.1093/brain/awaf033
Plasma phosphorylated tau217 strongly associates with memory deficits in the Alzheimer's disease spectrum
Abstract
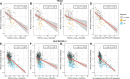
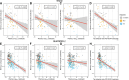
Plasma phosphorylated tau (p-tau) biomarkers open unprecedented opportunities for identifying carriers of Alzheimer's disease pathophysiology in early disease stages using minimally invasive techniques. Plasma p-tau biomarkers are believed to reflect tau phosphorylation and secretion. However, it remains unclear to what extent the magnitude of plasma p-tau abnormalities reflects neuronal network disturbance in the form of cognitive impairment. To address this question, we included 103 cognitively unimpaired elderly and 40 cognitively impaired, amyloid-β-positive individuals from the TRIAD cohort, in addition to 336 cognitively unimpaired and 216 cognitively impaired, amyloid-β-positive older adults from the BioFINDER-2 cohort. Participants had tau PET scans, amyloid PET scans or amyloid CSF, p-tau217, p-tau181 and p-tau231 blood measures, structural T1-MRI and cognitive assessments. In this cross-sectional study, we used regression models and correlation analyses to assess the relationship between plasma biomarkers and cognitive scores. Furthermore, we applied receiver operating characteristic curves to assess cognitive impairment across plasma biomarkers. Finally, we categorized participants into amyloid (A), p-tau (T1) and tau PET (T2) positive (+) or negative (-) profiles and ran non-parametric comparisons to assess differences across cognitive domains. We found that plasma p-tau217 was more associated with cognitive performance than p-tau181 and p-tau231 and that this relationship was particularly strong for memory scores (TRIAD: βp-tau217 = -0.53, βp-tau181 = -0.35 and βp-tau231 = -0.24; BioFINDER-2: βp-tau217 = -0.52, βp-tau181 = -0.24 and βp-tau231 = -0.29). Associations in amyloid-β-positive participants resembled these results, but other cognitive scores also showed strong associations in cognitively impaired individuals. Moreover, plasma p-tau217 outperformed plasma p-tau181 and plasma p-tau231 in identifying memory impairment (area under the curve values for TRIAD: p-tau217 = 0.86, p-tau181 = 0.77 and p-tau231 = 0.75; and for BioFINDER-2: p-tau217 = 0.86, p-tau181 = 0.76 and p-tau231 = 0.81) and in identifying executive function impairment only in the BioFINDER-2 cohort (p-tau217 = 0.82, p-tau181 = 0.76 and p-tau231 = 0.76). Lastly, we showed that subtle memory deficits were present in A+T1+T2- participants for plasma p-tau217 (P = 0.007) and plasma p-tau181 (P = 0.01) in the TRIAD cohort and for all biomarkers across cognitive domains in A+T1+T2- and A+T1+T2- individuals (P < 0.001 in all) in the BioFINDER-2 cohort. The A+T1+T2- individuals showed cognitive deficits in both cohorts (P < 0.001 in all). Together, our results suggest that plasma p-tau217 stands out as a biomarker capable of identifying memory deficits attributable to Alzheimer's disease and that memory impairment certainly occurs in amyloid-β- and plasma p-tau-positive individuals who have no significant amounts of tau in the neocortex.
Keywords: Alzheimer’s disease; memory; plasma p-tau217; tau discordance.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
G.S. has received speaker fees from Springer and Adium. G.T.-B. receives salary and stock from Johnson & Johnson. He also holds patents for Janssen Simoa assays CSF p217+Tau, Plasma p217+Tau, and plasma CD tTau assays, respectively. S.P. has acquired research support (for the institution) from Avid and ki elements through ADDF. In the past 2 years, he has received consultancy/speaker fees from BioArtic, Biogen, Eisai, Eli Lilly, Novo Nordisk, and Roche. H.Z. has served at scientific advisory boards and/or as a consultant for AbbVie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, Merry Life, NervGen, Novo Nordisk, OptoCeutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures in symposia sponsored by AlzeCure, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, and Roche; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and at advisory boards for AbbVie, AC Immune, ALZpath, AriBio, Beckman Coulter, BioArctic, Biogen, Eisai, Lilly, Moleac Pte Ltd, Neurimmune, Novartis, Ono Pharma, Prothena, Quanterix, Roche Diagnostics, Sanofi and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programmes for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. O.H. is an employee of Eli Lilly and Lund University. The other authors have no competing interests to disclose.
Figures



References
-
- Hampel H, Buerger K, Zinkowski R, et al. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: A comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. - PubMed
-
- Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature. 2018;554:249–254. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

