Caffeine ameliorates metabolic-associated steatohepatitis by rescuing hepatic Dusp9
- PMID: 39879738
- PMCID: PMC11815699
- DOI: 10.1016/j.redox.2025.103499
Caffeine ameliorates metabolic-associated steatohepatitis by rescuing hepatic Dusp9
Abstract
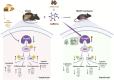
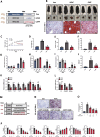
Caffeine (CAFF) is abundant in black coffee. As one of the most widely consumed beverages globally, coffee has been the focus of increasing clinical and basic research, particularly regarding its benefits in alleviating metabolic dysfunction-associated steatotic liver disease (MASLD). However, the therapeutic effects of CAFF on metabolic-associated steatohepatitis (MASH) and the underlying mechanisms remain unclear. In this study, we demonstrated that CAFF potently reduced hepatic steatosis, inflammation, and early-stage liver fibrosis in MASH mice induced by prolonged (36 weeks) high-fat high-carbohydrate (HFHC) diets and high-fat diets combined with carbon tetrachloride (CCl4) injections. By using multiple target-identifying strategies, including surface plasmon resonance (SPR), cellular thermal shift assay (CETSA), and drug affinity responsive target stability (DARTS) assay, we identified dual-specificity phosphatase 9 (Dusp9) as a key therapeutic target, which was diminished by HFHC but restored with CAFF treatment. Dusp9 knockdown in vivo and in vitro exacerbated glycolipid metabolism disorders and stunningly counteracted the systemic therapeutic effects of CAFF in the MASH models. In addition, CAFF inactivated the ASK1-p38/JNK, a downstream signaling pathway of Dusp9, which regulates inflammation and apoptosis. Our study highlights the multifaceted benefits of CAFF in treating MASH by rescuing hepatic Dusp9 expression, thereby reversing glycolipid metabolism disorders, liver inflammation, and fibrosis. These findings provide experimental evidence supporting the clinical and daily use of CAFF and black coffee in managing MASH patients.
Keywords: Black coffee; Caffeine (CAFF); Dual-specificity phosphatase 9 (Dusp9); Liver fibrosis; Metabolic dysfunction-associated steatotic liver disease (MASLD).
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors disclose no conflicts of interest.
Figures







References
-
- Lazarus J.V., et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022;19(1):60–78. - PubMed
-
- Yip T.C., et al. Asian perspective on NAFLD-associated HCC. J. Hepatol. 2022;76(3):726–734. - PubMed
-
- Targher G., et al. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat. Rev. Gastroenterol. Hepatol. 2021;18(9):599–612. - PubMed
-
- En Li Cho E., et al. Global prevalence of non-alcoholic fatty liver disease in type 2 diabetes mellitus: an updated systematic review and meta-analysis. Gut. 2023;72(11):2138–2148. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

