Improved Growth Velocity Using a New Liquid Human Milk Fortifier in Very Low Birth Weight Infants: A Multicenter, Retrospective Study
- PMID: 39880007
- PMCID: PMC12431800
- DOI: 10.1055/a-2527-4638
Improved Growth Velocity Using a New Liquid Human Milk Fortifier in Very Low Birth Weight Infants: A Multicenter, Retrospective Study
Abstract
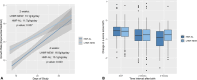
This study aimed to compare growth outcomes and tolerance among very low birth weight (VLBW) infants receiving a new, liquid human milk fortifier (LHMF-NEW) or a human milk fortifier-acidified liquid (HMF-AL).Retrospective, multicenter study of 515 VLBW infants in three regional neonatal intensive care units. The primary objective was to compare growth velocity (g/kg/d) during fortification between groups by repeated measures regression. Secondary outcomes of interest were feeding tolerance and the incidence of late-onset sepsis, necrotizing enterocolitis, and metabolic acidosis. Student's t, analysis of variance, Wilcoxon, and Kruskal-Wallis tests were used for numeric variables, or chi-squared and Fisher's exact test for categorical variables.No demographic differences were identified between the groups (HMF-AL, n = 242; LHMF-NEW, n = 273). Growth velocity during fortification was significantly higher in the group receiving LHMF-NEW, despite relatively similar total fluid, calorie, or protein intake (p = 0.001). Feeding intolerance was comparable between fortifiers. Necrotizing enterocolitis and late-onset sepsis did not differ between groups and metabolic acidosis was diagnosed less frequently with the LHMF-NEW. Anthropometric measures at discharge and length of stay were comparable.Infants receiving human milk fortified with the LHMF-NEW had faster growth velocity during fortification, similar tolerance, and less metabolic acidosis compared with an earlier cohort of infants who received human milk fortified with an HMF-AL. · Among VLBW infants, using an LHMF-NEW resulted in a faster growth velocity in weight during several weeks of fortification than using the previous HMF-AL.. · The incidence of feeding intolerance (stopping feeds >8 hour) in any given week of fortification was low and not different between groups. Also, late-onset sepsis and necrotizing enterocolitis were uncommon with no differences between groups, whereas the incidence of metabolic acidosis was lower in infants receiving the LHMF-NEW.. · No differences in length of stay or anthropometrics at discharge were identified..
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
F.M., J.F., and A.F. are part of Mead Johnson Nutrition Speaker's Bureau. F.M. is a recipient of grant funding from Mead Johnson Nutrition.
Figures

References
-
- Section on Breastfeeding, Committee on Nutrition, Committee on Fetus and Newborn . Parker M G, Stellwagen L M, Noble L, Kim J H, Poindexter B B, Puopolo K M. Promoting human milk and breastfeeding for the very low birth weight infant. Pediatrics. 2021;148(05):e2021054272. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

