Rehabilitation using virtual gaming for Hospital and hOMe-Based training for the Upper limb in acute and subacute Stroke (RHOMBUS II): results of a feasibility randomised controlled trial
- PMID: 39880460
- PMCID: PMC11781105
- DOI: 10.1136/bmjopen-2024-089672
Rehabilitation using virtual gaming for Hospital and hOMe-Based training for the Upper limb in acute and subacute Stroke (RHOMBUS II): results of a feasibility randomised controlled trial
Abstract
Objective: To investigate the safety, feasibility and acceptability of the Neurofenix platform for upper-limb rehabilitation in acute and subacute stroke.
Design: A feasibility randomised controlled trial with a parallel process evaluation.
Setting: Acute Stroke Unit and participants' homes (London, UK).
Participants:
24 adults (
Interventions: Participants randomised to the intervention or control group on a 2:1 ratio. The intervention group (n=16) received usual care plus the Neurofenix platform for 7 weeks. The control group (n=8) received usual care only.
Outcomes: Safety was assessed through adverse events (AEs), pain, spasticity and fatigue. Feasibility was assessed through training and support requirements and intervention fidelity. Acceptability was assessed through a satisfaction questionnaire. Impairment, activity and participation outcomes were also collected at baseline and 7 weeks to assess their suitability for use in a definitive trial.
Randomisation: Computer-generated, allocation sequence concealed by opaque, sealed envelopes.
Blinding: Participants and assessors were not blinded; statistician blinded for data processing and analysis.
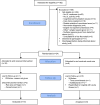
Results: 192 stroke survivors were screened for eligibility, and 24 were recruited and randomised. Intervention group: n=16, mean age 66.5 years; median 9.5 days post stroke.
Control group: n=8, mean age 64.6 years; median 17.5 days post stroke. Three participants withdrew before the 7-week assessment, n=21 included in the analysis (intervention group n=15; control group n=6). No significant group differences in fatigue, spasticity, pain scores or total number of AEs. The median (IQR) time to train participants was 98 (64) min over 1-3 sessions. Participants trained with the platform for a median (range) of 11 (1-58) hours, equating to 94 min extra per week. The mean satisfaction score was 34.9 out of 40.
Conclusion: The Neurofenix platform is safe, feasible and well accepted as an adjunct to usual care in acute and subacute stroke rehabilitation. There was a wide range of engagement with the platform in a cohort of stroke survivors which was varied in age and level of impairment. Recruitment, training and support were manageable and completion of data was good, indicating that a future randomised controlled trial would be feasible.
Trial registration number: ISRCTN11440079.
Keywords: REHABILITATION MEDICINE; Stroke; Telemedicine.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: DAA, KB and GS-B are employed by Neurofenix (UK and USA), a technology-enabled neuro-rehabilitation provider developing a remote VR platform including sensor-based medical devices for neurological patients. Neurofenix provided the VR platforms and technical support to the research therapists. Neurofenix had no influence on the design of the study, data collection, analysis and interpretation of the data or manuscript preparation. All other authors have no competing interest to declare.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
