Arginase-1-specific T cells target and modulate tumor-associated macrophages
- PMID: 39880485
- PMCID: PMC11781113
- DOI: 10.1136/jitc-2024-009930
Arginase-1-specific T cells target and modulate tumor-associated macrophages
Abstract
Background: Arginase-1 (Arg1) expressing tumor-associated macrophages (TAMs) may create an immune-suppressive tumor microenvironment (TME), which is a significant challenge for cancer immunotherapy. We previously reported the existence of Arg1-specific memory T cells among peripheral blood mononuclear cells (PBMCs) and described that Arg-1-based immune modulatory vaccines (IMVs) control tumor growth and alter the M1/M2 macrophage ratio in murine models of cancer. In the present study, we investigated how Arg1-specific T cells can directly target TAMs and influence their polarization.
Methods: Murine Arg1-specific CD4+T cells isolated from splenocytes of animals vaccinated with an Arg1-derived peptide in the adjuvant montanide were co-cultured with either in vitro M2-differentiated bone marrow-derived macrophages or ex vivo isolated F4/80+TAMs. Human Arg1-specific CD4+T cell clones were co-cultured with Arg1-expressing TAMs generated in vitro from either PBMC-derived CD14+cells or the myeloid cell lines MonoMac1 and THP-1. MHC class II-restricted Arg-1 peptide presentation by macrophages was confirmed by immunopeptidomics. T-cell-mediated changes in the macrophage immune phenotype and cytokine microenvironment were examined using flow cytometry, RT-qPCR and multiplex immunoassay. The effect of Arg1-derived peptide IMV on TAMs in vivo was assessed by multiplex gene analysis of F4/80+cells.
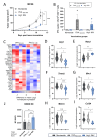
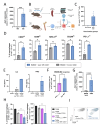
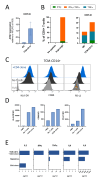
Results: We show that Arg1-based IMV-mediated tumor control was linked to a decrease in multiple immunosuppressive pathways in the TAM population of the treated animals. Tumor-conditioned media (TCM) derived from Arg1-vaccinated mice induced significantly higher upregulation of MHC-II on exposed myeloid cells compared with controls. Furthermore, murine CD4+Arg1-specific T cells were able to target TAMs and effectively reprogram their phenotype ex vivo by secreting IL2 and IFNγ. Next, we established that human Arg1+TAMs present Arg1-derived peptides and are directly recognized by proinflammatory CD4+Arg1-specific T cell clones. These CD4+Arg1-specific T cells were able to reprogram TCM-conditioned macrophages as observed by increased expression of CD80 and HLA-DR.
Conclusions: TAMs may be directly targeted and modulated by Arg1-specific CD4+T cells. These findings provide a strong rationale for future clinical development of Arg1-based IMVs to alter the immune-suppressive TME by reprogramming TAMs and promoting a proinflammatory TME.
Keywords: Immune modulatory; Macrophage; T cell; Vaccine.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: MHA has made an invention based on the use of Arg1 for vaccinations. The rights of the invention have been transferred to Copenhagen University Hospital Herlev, according to the Danish Law of Public Inventions at Public Research Institutions. The capital region has licensed the rights to the company IO Biotech ApS. The patent application was filed by IO Biotech ApS. MHA is an advisor and shareholder at IO Biotech. EM, IL, MC, and AWP are employees at IO Biotech. The additional authors do not declare any competing financial interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
