Hawkmoth Pheromone Transduction Involves G-Protein-Dependent Phospholipase Cβ Signaling
- PMID: 39880675
- PMCID: PMC11964160
- DOI: 10.1523/ENEURO.0376-24.2024
Hawkmoth Pheromone Transduction Involves G-Protein-Dependent Phospholipase Cβ Signaling
Abstract
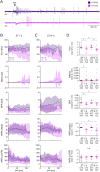
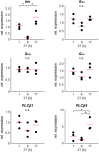
Evolutionary pressures adapted insect chemosensation to their respective physiological needs and tasks in their ecological niches. Solitary nocturnal moths rely on their acute olfactory sense to find mates at night. Pheromones are detected with maximized sensitivity and high temporal resolution through mechanisms that are mostly unknown. While the inverse topology of insect olfactory receptors and heteromerization with the olfactory receptor coreceptor suggest ionotropic transduction via odorant-gated receptor-ion channel complexes, contradictory data propose amplifying G-protein-coupled transduction. Here, we used in vivo tip-recordings of pheromone-sensitive sensilla of male Manduca sexta hawkmoths at specific times of day (rest vs activity). Since the olfactory receptor neurons distinguish signal parameters in three consecutive temporal windows of their pheromone response (phasic; tonic; late, long-lasting), respective response parameters were analyzed separately. Disruption of G-protein-coupled transduction and block of phospholipase C decreased and slowed the phasic response component during the activity phase of hawkmoths without affecting any other component of the response during activity and rest. A more targeted disruption of Gα subunits by blocking Gαo or sustained activation of Gαs using bacterial toxins affected the phasic pheromone response, while toxins targeting Gαq and Gα12/13 were ineffective. Consistent with these data, the expression of phospholipase Cβ4 depended on zeitgeber time, which indicates circadian clock-modulated metabotropic pheromone transduction cascades that maximize sensitivity and temporal resolution of pheromone transduction during the hawkmoth's activity phase. Thus, discrepancies in the literature on insect olfaction may be resolved by considering circadian timing and the distinct odor response components.
Keywords: circadian clock; insect; olfaction.
Copyright © 2025 Schneider et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





Similar articles
-
In situ tip-recordings found no evidence for an Orco-based ionotropic mechanism of pheromone-transduction in Manduca sexta.PLoS One. 2013 May 3;8(5):e62648. doi: 10.1371/journal.pone.0062648. Print 2013. PLoS One. 2013. PMID: 23671617 Free PMC article.
-
No Evidence for Ionotropic Pheromone Transduction in the Hawkmoth Manduca sexta.PLoS One. 2016 Nov 9;11(11):e0166060. doi: 10.1371/journal.pone.0166060. eCollection 2016. PLoS One. 2016. PMID: 27829053 Free PMC article.
-
The Diacylglycerol Analogs OAG and DOG Differentially Affect Primary Events of Pheromone Transduction in the Hawkmoth Manduca sexta in a Zeitgebertime-Dependent Manner Apparently Targeting TRP Channels.Front Cell Neurosci. 2018 Jul 24;12:218. doi: 10.3389/fncel.2018.00218. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30087596 Free PMC article.
-
Access to the odor world: olfactory receptors and their role for signal transduction in insects.Cell Mol Life Sci. 2018 Feb;75(3):485-508. doi: 10.1007/s00018-017-2627-5. Epub 2017 Aug 21. Cell Mol Life Sci. 2018. PMID: 28828501 Free PMC article. Review.
-
Working range of stimulus flux transduction determines dendrite size and relative number of pheromone component receptor neurons in moths.Chem Senses. 2012 May;37(4):299-313. doi: 10.1093/chemse/bjr122. Epub 2012 Jan 9. Chem Senses. 2012. PMID: 22230170 Review.
References
-
- Altner H, Prillinger L (1980) Ultrastructure of invertebrate chemo-, thermo-, and hygroreceptors and its functional significance. In: International review of cytology (Bourne GH, Danielli JF, eds), pp 69–139. New York: Academic Press.
-
- Baker TC, Cardé RT (1979) Analysis of pheromone-mediated behaviors in male Grapholita molesta, the oriental fruit moth (Lepidoptera: Tortricidae). Environ Entomol 8:956–968. 10.1093/ee/8.5.956 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
