Engineered heart muscle allografts for heart repair in primates and humans
- PMID: 39880949
- PMCID: PMC11903342
- DOI: 10.1038/s41586-024-08463-0
Engineered heart muscle allografts for heart repair in primates and humans
Abstract
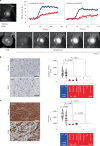
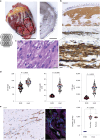
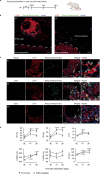
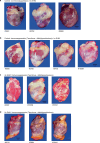
Cardiomyocytes can be implanted to remuscularize the failing heart1-7. Challenges include sufficient cardiomyocyte retention for a sustainable therapeutic impact without intolerable side effects, such as arrhythmia and tumour growth. We investigated the hypothesis that epicardial engineered heart muscle (EHM) allografts from induced pluripotent stem cell-derived cardiomyocytes and stromal cells structurally and functionally remuscularize the chronically failing heart without limiting side effects in rhesus macaques. After confirmation of in vitro and in vivo (nude rat model) equivalence of the newly developed rhesus macaque EHM model with a previously established Good Manufacturing Practice-compatible human EHM formulation8, long-term retention (up to 6 months) and dose-dependent enhancement of the target heart wall by EHM grafts constructed from 40 to 200 million cardiomyocytes/stromal cells were demonstrated in macaques with and without myocardial infarction-induced heart failure. In the heart failure model, evidence for EHM allograft-enhanced target heart wall contractility and ejection fraction, which are measures for local and global heart support, was obtained. Histopathological and gadolinium-based perfusion magnetic resonance imaging analyses confirmed cell retention and functional vascularization. Arrhythmia and tumour growth were not observed. The obtained feasibility, safety and efficacy data provided the pivotal underpinnings for the approval of a first-in-human clinical trial on tissue-engineered heart repair. Our clinical data confirmed remuscularization by EHM implantation in a patient with advanced heart failure.
© 2025. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests: W.-H.Z. is the founder, equity holder and advisor of Repairon GmbH. M.T. is advisor of Repairon GmbH. Repairon is working towards market authorization of EHM as advanced therapy medicinal product for applications in heart failure. Repairon had no influence on the design, conduct and interpretation of the study. The other authors declare no competing interests.
Figures













References
-
- Laflamme, M. A. et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol.25, 1015–1024 (2007). - PubMed
-
- Soonpaa, M. H., Koh, G. Y., Klug, M. G. & Field, L. J. Formation of nascent intercalated disks between grafted fetal cardiomyocytes and host myocardium. Science264, 98–101 (1994). - PubMed
-
- Muller-Ehmsen, J. et al. Rebuilding a damaged heart: long-term survival of transplanted neonatal rat cardiomyocytes after myocardial infarction and effect on cardiac function. Circulation105, 1720–1726 (2002). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

