Evaluation of the Digital Ventilated Cage® system for circadian phenotyping
- PMID: 39880968
- PMCID: PMC11779816
- DOI: 10.1038/s41598-025-87530-6
Evaluation of the Digital Ventilated Cage® system for circadian phenotyping
Abstract
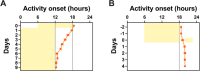
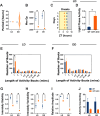
The study of circadian rhythms has been critically dependent upon analysing mouse home cage activity, typically employing wheel running activity under different lighting conditions. Here we assess a novel method, the Digital Ventilated Cage (DVC®, Tecniplast SpA, Italy), for circadian phenotyping. Based upon capacitive sensors mounted under black individually ventilated cages with inbuilt LED lighting, each cage becomes an independent light-controlled chamber. Home cage activity in C57BL/6J mice was recorded under a range of lighting conditions, along with circadian clock-deficient cryptochrome-deficient mice (Cry1-/-, Cry2-/- double knockout). C57BL/6J mice exhibited a 24 h period under light/dark conditions, with a free-running period of 23.5 h under constant dark, and period lengthening under constant light. Animals displayed expected phase shifting responses to jet-lag and nocturnal light pulses. Sex differences in circadian parameters and phase shifting responses were also observed. Cryptochrome-deficient mice showed subtle changes in activity under light/dark conditions and were arrhythmic under constant dark, as expected. Our results show the suitability of the DVC system for circadian behavioural screens, accurately detecting circadian period, circadian disruption, phase shifts and mice with clock defects. We provide an evaluation of the strengths and limitations of this method, highlighting how the use of the DVC for studying circadian rhythms depends upon the research requirements of the end user.
Keywords: Circadian disruption; Circadian phenotyping; Circadian screen; Home cage monitoring; Individually ventilated cage; Locomotor activity.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors (ST and SNP) have attended meetings funded by Tecniplast. However, Tecniplast had no involvement in the study design, data interpretation, or conclusions drawn from the research findings. As such, the authors declare no conflict of interest regarding the use of the DVC system. Ethics statement: The animal study was reviewed and approved by the Clinical Medicine Animal Welfare and Ethical Review Body (AWERB), University of Oxford.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

