Niche-derived Semaphorin 4A safeguards functional identity of myeloid-biased hematopoietic stem cells
- PMID: 39881190
- PMCID: PMC12025894
- DOI: 10.1038/s43587-024-00798-7
Niche-derived Semaphorin 4A safeguards functional identity of myeloid-biased hematopoietic stem cells
Erratum in
-
Author Correction: Niche-derived Semaphorin 4A safeguards functional identity of myeloid-biased hematopoietic stem cells.Nat Aging. 2025 Apr;5(4):720. doi: 10.1038/s43587-025-00837-x. Nat Aging. 2025. PMID: 39979638 No abstract available.
Abstract
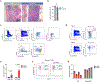
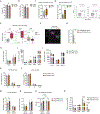
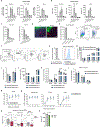
Somatic stem cell pools comprise diverse, highly specialized subsets whose individual contribution is critical for the overall regenerative function. In the bone marrow, myeloid-biased hematopoietic stem cells (myHSCs) are indispensable for replenishment of myeloid cells and platelets during inflammatory response but, at the same time, become irreversibly damaged during inflammation and aging. Here we identify an extrinsic factor, Semaphorin 4A (Sema4A), which non-cell-autonomously confers myHSC resilience to inflammatory stress. We show that, in the absence of Sema4A, myHSC inflammatory hyper-responsiveness in young mice drives excessive myHSC expansion, myeloid bias and profound loss of regenerative function with age. Mechanistically, Sema4A is mainly produced by neutrophils, signals via a cell surface receptor, Plexin D1, and safeguards the myHSC epigenetic state. Our study shows that, by selectively protecting a distinct stem cell subset, an extrinsic factor preserves functional diversity of somatic stem cell pool throughout organismal lifespan.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: L.S., S. Radtke and H.P.-K. are listed inventors on patent application 18/717,971 relating to this work. All other authors declare no competing interests.
Figures









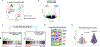
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

