SLC25A1 and ACLY maintain cytosolic acetyl-CoA and regulate ferroptosis susceptibility via FSP1 acetylation
- PMID: 39881208
- PMCID: PMC11914110
- DOI: 10.1038/s44318-025-00369-5
SLC25A1 and ACLY maintain cytosolic acetyl-CoA and regulate ferroptosis susceptibility via FSP1 acetylation
Abstract
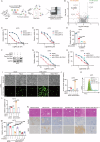
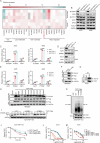
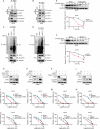
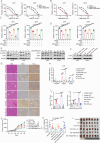
Ferroptosis, an iron-dependent form of programmed cell death characterized by excessive lipid hydroperoxides accumulation, emerges as a promising target in cancer therapy. Among the solute carrier (SLC) superfamily, the cystine/glutamate transporter system antiporter components SLC3A2 and SLC7A11 are known to regulate ferroptosis by facilitating cystine import for ferroptosis inhibition. However, the contribution of additional SLC superfamily members to ferroptosis remains poorly understood. Here, we use a targeted CRISPR-Cas9 screen of the SLC superfamily to identify SLC25A1 as a critical ferroptosis regulator in human cancer cells. SLC25A1 drives citrate export from the mitochondria to the cytosol, where it fuels acetyl-CoA synthesis by ATP citrate lyase (ACLY). This acetyl-CoA supply sustains FSP1 acetylation and prevents its degradation by the proteasome via K29-linked ubiquitin chains. K168 is the primary site of FSP1 acetylation and deacetylation by KAT2B and HDAC3, respectively. Pharmacological inhibition of SLC25A1 and ACLY significantly enhances cancer cell susceptibility to ferroptosis both in vitro and in vivo. Targeting the SLC25A1-ACLY axis is therefore a potential therapeutic strategy for ferroptosis-targeted cancer intervention.
Keywords: ACLY; Acetylation; FSP1; Ferroptosis; SLC25A1.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 2022YFA0912600/MOST | National Science and Technology Infrastructure Program (National Key Science Projects Program)
- 2023YFC2308200/MOST | National Science and Technology Infrastructure Program (National Key Science Projects Program)
- 32100579/MOST | National Natural Science Foundation of China (NSFC)
- 82341011/MOST | National Natural Science Foundation of China (NSFC)
- 32370800/MOST | National Natural Science Foundation of China (NSFC)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

