Antibiotic duration for common bacterial infections-a systematic review
- PMID: 39881797
- PMCID: PMC11775593
- DOI: 10.1093/jacamr/dlae215
Antibiotic duration for common bacterial infections-a systematic review
Abstract
Background: Reducing antibiotic duration is a key stewardship intervention to mitigate antimicrobial resistance (AMR). We examined current evidence informing antibiotic duration for common bacterial infections to identify any gaps in terms of settings, patient populations and infectious conditions. Trial methodologies were assessed to identify areas for improvement.
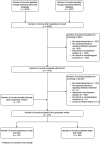
Methods: MEDLINE and Embase were searched up to July 2024 for randomized trials comparing antibiotic durations in hospital and community settings (PROSPERO 2021, CRD42021276209). A narrative synthesis of the results was performed with a review on the major guidelines published by IDSA, NICE, WHO and other international societies to assess the impact of these trials on practice guidance.
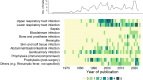
Results: Out of 315 studies, 85% concluded equivalence or non-inferiority of shorter courses. Adult bacterial sinusitis, community-acquired pneumonia, female cystitis/pyelonephritis, uncomplicated cellulitis and intra-abdominal infection with adequate source control and perioperative prophylaxis had robust evidence supporting shorter durations. Few trials studied severe infections, such as bloodstream infections and ventilator-associated pneumonia. Twenty-three (7%) of the trials were conducted in intensive care settings and only 43 trials (14%) enrolled patients from low-to-middle- or low-income countries. Only 15% of studies were at low risk for bias.
Conclusions: Reducing antibiotic duration likely remains an important strategy for antibiotic stewardship, and an area of active research. While shorter antibiotic courses may be suitable for many bacterial infections, more evidence is needed for severe infections and in low- and middle-income settings.
© The Author(s) 2025. Published by Oxford University Press on behalf of British Society for Antimicrobial Chemotherapy.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
