Mechanisms of vagus nerve stimulation for the treatment of neurodevelopmental disorders: a focus on microglia and neuroinflammation
- PMID: 39881804
- PMCID: PMC11774973
- DOI: 10.3389/fnins.2024.1527842
Mechanisms of vagus nerve stimulation for the treatment of neurodevelopmental disorders: a focus on microglia and neuroinflammation
Abstract
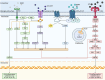
The vagus nerve (VN) is the primary parasympathetic nerve, providing two-way communication between the body and brain through a network of afferent and efferent fibers. Evidence suggests that altered VN signaling is linked to changes in the neuroimmune system, including microglia. Dysfunction of microglia, the resident innate immune cells of the brain, is associated with various neurodevelopmental disorders, including schizophrenia, attention deficit hyperactive disorder (ADHD), autism spectrum disorder (ASD), and epilepsy. While the mechanistic understanding linking the VN, microglia, and neurodevelopmental disorders remains incomplete, vagus nerve stimulation (VNS) may provide a better understanding of the VN's mechanisms and act as a possible treatment modality. In this review we examine the VN's important role in modulating the immune system through the inflammatory reflex, which involves the cholinergic anti-inflammatory pathway, which releases acetylcholine. Within the central nervous system (CNS), the direct release of acetylcholine can also be triggered by VNS. Homeostatic balance in the CNS is notably maintained by microglia. Microglia facilitate neurogenesis, oligodendrogenesis, and astrogenesis, and promote neuronal survival via trophic factor release. These cells also monitor the CNS microenvironment through a complex sensome, including groups of receptors and proteins enabling microglia to modify neuroimmune health and CNS neurochemistry. Given the limitations of pharmacological interventions for the treatment of neurodevelopmental disorders, this review seeks to explore the application of VNS as an intervention for neurodevelopmental conditions. Accordingly, we review the established mechanisms of VNS action, e.g., modulation of microglia and various neurotransmitter pathways, as well as emerging preclinical and clinical evidence supporting VNS's impact on symptoms associated with neurodevelopmental disorders, such as those related to CNS inflammation induced by infections. We also discuss the potential of adapting non-invasive VNS for the prevention and treatment of these conditions. Overall, this review is intended to increase the understanding of VN's potential for alleviating microglial dysfunction involved in schizophrenia, ADHD, ASD, and epilepsy. Additionally, we aim to reveal new concepts in the field of CNS inflammation and microglia, which could serve to understand the mechanisms of VNS in the development of new therapies for neurodevelopmental disorders.
Keywords: acetylcholine; microglia; neurodevelopmental disorders; vagus nerve; vagus nerve stimulation.
Copyright © 2025 Gargus, Ben-Azu, Landwehr, Dunn, Errico and Tremblay.
Conflict of interest statement
Joseph P. Errico is a consultant for electroCore and a board member of the Vagus Nerve Society. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Aalbers M. W., Klinkenberg S., Rijkers K., Verschuure P., Kessels A., Aldenkamp A., et al. . (2012). The effects of Vagus nerve stimulation on pro-and anti-inflammatory cytokines in children with refractory epilepsy: An exploratory study. Neuroimmunomodulation 19, 352–358. doi: 10.1159/000341402, PMID: - DOI - PubMed
-
- Andero R., Choi D. C., Ressler K. J. (2014). “Chapter six - BDNF–TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders” in Progress in molecular biology and translational science [internet]. eds. Khan Z. U., Muly E. C. (Cambridge, MA: Academic Press; ), 169–192. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

