Efficient removal of Cr(iii) by microbially induced calcium carbonate precipitation
- PMID: 39882011
- PMCID: PMC11775500
- DOI: 10.1039/d4ra05829a
Efficient removal of Cr(iii) by microbially induced calcium carbonate precipitation
Abstract
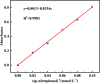
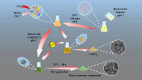
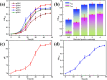
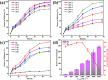
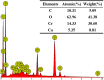
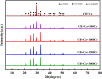
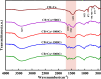
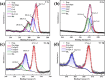
Microbially induced calcium carbonate precipitation (MICP) has emerged as a promising technique for environmental remediation, particularly for heavy metal removal. This study explores the potential of MICP for Cr(iii) removal, analyzing the effects of temperature, pH, calcium source addition, and initial Cr(iii) concentration on removal efficiency. The results show that Cr(iii) can be efficiently removed with a removal rate approaching 100% under optimal conditions (25 °C, pH 7.0, 1.0 g CaCl2). The presence of Cr(iii) induces the transformation of CaCO3 crystals from calcite to spherulitic aragonite, forming Cr-bearing carbonate compounds and hydroxides. This study provides insights into the mechanisms and optimal conditions for MICP-mediated Cr(iii) removal, highlighting its feasibility and effectiveness for large-scale environmental remediation and offering an economical and environmentally friendly solution to Cr contamination.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Different calcium sources affect the products and sites of mineralized Cr(VI) by microbially induced carbonate precipitation.Chemosphere. 2024 Sep;363:142977. doi: 10.1016/j.chemosphere.2024.142977. Epub 2024 Jul 29. Chemosphere. 2024. PMID: 39084306
-
Urease-catalyzed microbial and enzymatic carbonate precipitation for eco-friendly heavy metal remediation.Lett Appl Microbiol. 2025 Feb 3;78(2):ovaf022. doi: 10.1093/lambio/ovaf022. Lett Appl Microbiol. 2025. PMID: 39938921 Review.
-
Microbially induced carbonate precipitation with Arthrobacter creatinolyticus: An eco-friendly strategy for mitigation of chromium contamination.J Environ Manage. 2024 Aug;365:121300. doi: 10.1016/j.jenvman.2024.121300. Epub 2024 Jul 2. J Environ Manage. 2024. PMID: 38955041
-
A review on the applications of microbially induced calcium carbonate precipitation in solid waste treatment and soil remediation.Chemosphere. 2022 Mar;290:133229. doi: 10.1016/j.chemosphere.2021.133229. Epub 2021 Dec 8. Chemosphere. 2022. PMID: 34896177 Review.
-
Bioremediation of Heavy Metal-Contaminated Solution and Aged Refuse by Microbially Induced Calcium Carbonate Precipitation: Further Insights into Sporosarcina pasteurii.Microorganisms. 2025 Jan 2;13(1):64. doi: 10.3390/microorganisms13010064. Microorganisms. 2025. PMID: 39858832 Free PMC article.
Cited by
-
Response mechanism of extracellular polymers in the remediation of chromium pollution by carbonate mineralizing bacteria.RSC Adv. 2025 May 13;15(18):14227-14234. doi: 10.1039/d5ra01916h. eCollection 2025 Apr 28. RSC Adv. 2025. PMID: 40364817 Free PMC article.
References
-
- Ghangrekar M. M., Sathe S. M. and Chakraborty I., in Emerging Technologies in Environmental Bioremediation, 2020, pp. 233–255, 10.1016/b978-0-12-819860-5.00009-2 - DOI
-
- Kumar P. Nashath Omer S. Reddy M M. Saravanan P. Rajeshkannan R. Rajasimman M. Shanmugam V. K. Kamyab H. Gupta V. K. Vasseghian Y. J. Energy Inst. 2024;114:101626. doi: 10.1016/j.joei.2024.101626. - DOI
-
- Suresh G. Balasubramanian B. Ravichandran N. Ramesh B. Kamyab H. Velmurugan P. Siva G. V. Ravi A. V. Biomass Convers. Biorefin. 2021;11:383–391. doi: 10.1007/s13399-020-01259-y. - DOI
-
- Wang J. Wang P. Wang H. Dong J. Chen W. Wang X. Wang S. Hayat T. Alsaedi A. Wang X. ACS Sustain. Chem. Eng. 2017;5:7165–7174. doi: 10.1021/acssuschemeng.7b01347. - DOI
LinkOut - more resources
Full Text Sources

