Preparedness, prevention and control related to zoonotic avian influenza
- PMID: 39882189
- PMCID: PMC11775931
- DOI: 10.2903/j.efsa.2025.9191
Preparedness, prevention and control related to zoonotic avian influenza
Abstract
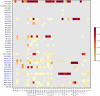
A risk assessment framework was developed to evaluate the zoonotic potential of avian influenza (AI), focusing on virus mutations linked to phenotypic traits related to mammalian adaptation identified in the literature. Virus sequences were screened for the presence of these mutations and their geographical, temporal and subtype-specific trends. Spillover events to mammals (including humans) and human seroprevalence studies were also reviewed. Thirty-four mutations associated with five phenotypic traits (increased receptor specificity, haemagglutinin stability, neuraminidase specificity, enhanced polymerase activity and evasion of innate immunity) were shortlisted. AI viruses (AIVs) carrying multiple adaptive mutations and traits belonged to both low and highly pathogenic subtypes, mainly to A(H9N2), A(H7N9), A(H5N6) and A(H3N8), were sporadic and primarily detected in Asia. In the EU/EEA, H5Nx viruses of clade 2.3.4.4b, which have increased opportunities for evolution due to widespread circulation in birds and occasional cases/outbreaks in mammals, have acquired the highest number of zoonotic traits. Adaptive traits, such as enhanced polymerase activity and immune evasion, were frequently acquired, while receptor-specific mutations remained rare. Globally, human cases remain rare, with the majority overall due to A(H5N1), A(H5N6), A(H7N9) and A(H9N2) that are among the subtypes that tend to have a higher number of adaptive traits. The main drivers of mammalian adaptation include virus and host characteristics, and external factors increasing AIV exposure of mammals and humans to wild and domestic birds (e.g. human activities and ecological factors). Comprehensive surveillance of AIVs targeting adaptive mutations with whole genome sequencing in animals and humans is essential for early detection of zoonotic AIVs and efficient implementation of control measures. All preparedness, preventive and control measures must be implemented under a One Health framework and tailored to the setting and the epidemiological situation; in particular, enhanced monitoring, biosecurity, genomic surveillance and global collaboration are critical for mitigating the zoonotic risks of AIV.
Keywords: avian influenza; birds; highly pathogenic avian influenza (HPAI); mammals; mutations; preparedness; public health.
© 2025 European Food Safety Authority. EFSA Journal published by Wiley‐VCH GmbH on behalf of European Food Safety Authority.
Figures





















References
-
- Agüero, M. , Monne, I. , Sánchez, A. , Zecchin, B. , Fusaro, A. , Ruano, M. J. , Del Valle Arrojo, M. , Fernández‐Antonio, R. , Souto, A. M. , Tordable, P. , Cañás, J. , Bonfante, F. , Giussani, E. , Terregino, C. , & Orejas, J. J. (2023). Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Eurosurveillance, 28, 2300001. 10.2807/1560-7917.ES.2023.28.3.2300001 - DOI - PMC - PubMed
-
- Aznar, E. , Casas, I. , González Praetorius, A. , Ruano Ramos, M. J. , Pozo, F. , Sierra Moros, M. J. , García Rivera, M. V. , Sánchez Sánchez, A. , García Villacieros, E. , Saravia, G. , Iglesias‐Caballero, M. , Román Marcos, E. , & García San Miguel, L. (2023). Influenza A(H5N1) detection in two asymptomatic poultry farm workers in Spain, September to October 2022: Suspected environmental contamination. Eurosurveillance, 28, 2300107. 10.2807/1560–7917.ES.2023.28.8.2300107 - DOI - PMC - PubMed
-
- BAuA (Bundesanstalt für Arbeitsschutz und Arbeitsmedizin) . (2007a). Beschluss 608: Empfehlung spezieller Maßnahmen zum Schutz der Beschäftigten vor Infektionen durch hochpathogene aviäre Influenzaviren (Klassische Geflügelpest, Vogelgrippe). Published in the Gemeinsames Ministerialblatt (GMBl). Bundesanstalt für Arbeitsschutz und Arbeitsmedizin, 19, 403–407. https://www.baua.de/DE/Angebote/Regelwerk/TRBA/Beschluss‐608
LinkOut - more resources
Full Text Sources
