Suppression of MCP-1, IFN-γ and IL-6 production of HNSCC ex vivo by pembrolizumab added to docetaxel and cisplatin (TP) exceeding those of TP alone is linked to improved survival
- PMID: 39882242
- PMCID: PMC11774711
- DOI: 10.3389/fimmu.2024.1473897
Suppression of MCP-1, IFN-γ and IL-6 production of HNSCC ex vivo by pembrolizumab added to docetaxel and cisplatin (TP) exceeding those of TP alone is linked to improved survival
Abstract
Background: Adding pembrolizumab, an anti-PD-1 antibody approved for treatment of head and neck squamous cell carcinoma (HNSCC) to neoadjuvant (induction-) chemotherapy utilizing docetaxel and cisplatin (TP) followed by radiotherapy may improve outcome in larynx organ-preservation (LOP) that is investigated in the European Larynx-Organ preservation Study (ELOS). As biomarkers for response to TP and pembrolizumab +TP are missing but may include cytokines, this work aims on determining cytokines potentially linked to outcome as prognostic markers sufficient to predict and/or monitor response to successful LOP.
Methods: Collagenase IV digests were generated from 47 histopathological confirmed HNSCC tumor samples and seeded in 96-well plates containing pembrolizumab, docetaxel, cisplatin either solely or in binary or ternary combination. According to the FLAVINO protocol, supernatants were collected after 3 days, adherent cells fixed using ethanol, air-dried and pan-cytokeratin positive epithelial cells counted using fluorescence microscopy. The cytokines IL-6, IL-8, IFN-γ, IP-10, MCP-1, TNF-α, and VEGF in the supernatant were quantified by sandwich ELISA.
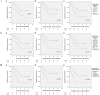
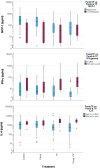
Results: The mode of interaction between pembrolizumab and TP was assessed and correlated to outcome (overall, disease-specific and progression-free survival of patients). Suppression of MCP-1, IFN-γ and IL-6 production by pembrolizumab + TP exceeding the suppressive effect of TP was detected in the majority of samples and linked to improved survival. Multivariate Cox proportional hazard regression modeling revealed MCP-1, IFN-γ and IL-6 as independent outcome predictors.
Conclusions: Comparing response to TP vs. pembrolizumab vs. TP + pembrolizumab may allow for identification of patients with superior outcome independent from treatment applied.
Keywords: CCL2); PD-1:PD-L1 immune-checkpoint inhibitor (ICI) pembrolizumab; biomarker research; local and locoregional advanced head and neck squamous cell carcinoma (HNSCC); monocyte chemoattractant protein 1 (MCP-1; neoadjuvant (induction) chemotherapy; predictive assay for chemoresponse-evaluation.
Copyright © 2025 Wellhausen, Röhl, Berszin, Krücken, Zebralla, Pirlich, Stoehr, Wiegand, Dietz, Wald and Wichmann.
Conflict of interest statement
AD received funding for research the ELOS randomized controlled larynx-organ preservation trial. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Dietz A, Wichmann G, Kuhnt T, Pfreundner L, Hagen R, Scheich M, et al. Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann Oncol. (2018) 29:2105–14. doi: 10.1093/annonc/mdy332 - DOI - PubMed
-
- Wichmann G, Krüger A, Boehm A, Kolb M, Hofer M, Fischer M, et al. Induction chemotherapy followed by radiotherapy for larynx preservation in advanced laryngeal and hypopharyngeal cancer: Outcome prediction after one cycle induction chemotherapy by a score based on clinical evaluation, computed tomography-based volumetry and 18F-FDG-PET/CT. Eur J Cancer. (2017) 72:144–55. doi: 10.1016/j.ejca.2016.11.013 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

