Characterization of LTBP2 mutation causing mitral valve prolapse
- PMID: 39882270
- PMCID: PMC11775471
- DOI: 10.1093/ehjopen/oeae106
Characterization of LTBP2 mutation causing mitral valve prolapse
Abstract
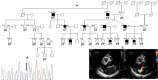
Aims: Mitral valve prolapse (MVP) is a common valvular disorder associated with significant morbidity and mortality, with a strong genetic basis. This study aimed to identify a mutation in a family with MVP and to characterize the valve phenotype in LTBP2 knockout (KO) mice.
Methods and results: Exome sequencing and segregation analysis were performed on a large family with MVP. Two mouse strains were generated: a complete KO of the LTBP2 gene and a knockin (KI) of the human mutation. At 6 months, phenotyping was conducted using echocardiography, histology, eye optical coherence tomography, and quantitative polymerase chain reaction analysis for TGF-β signalling targets (periostin/POSTN, RUNX2, and CTGF) in valve tissues. LTBP2 rs117800773 V1506M mutation exhibited segregation with MVP. LTBP2 KO mice had a higher incidence of myxomatous changes by histology (7 of 9 of KO vs. 0 of 7 control animals, P = 0.00186) and echocardiography (7 of 9 vs. 0 of 8, P = 0.0011). LTBP2 KI mice for the human mutation showed a significantly elevated myxomatous histological phenotype (8 of 8 vs. 0 of 9, P = 0.00004) as well as by echocardiography (6 of 8 vs. 0 of 9, P = 0.00123). Knockout mice demonstrated an increase in the depth of the anterior chamber as well as reduced visual acuity. LTBP2 KO mice demonstrated overexpression of both TGF-β signalling targets RUNX2 and periostin (P = 0.0144 and P = 0.001826, respectively).
Conclusion: We report a KO mouse strain with an LTBP2 mutation, demonstrating a valve phenotype, alongside a family with a novel mutation linked to MVP.
Keywords: LTBP2; Mitral valve prolapse; Myxomatous valve.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared.
Figures



References
-
- Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7. - PubMed
-
- Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, Horton K, Ogunyankin KO, Palma RA, Velazquez EJ. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32:1–64. - PubMed
-
- Levine RA, Hagége AA, Judge DP, Padala M, Dal-Bianco JP, Aikawa E, Beaudoin J, Bischoff J, Bouatia-Naji N, Bruneval P, Butcher JT, Carpentier A, Chaput M, Chester AH, Clusel C, Delling FN, Dietz HC, Dina C, Durst R, Fernandez-Friera L, Handschumacher MD, Jensen MO, Jeunemaitre XP, Le Marec H, Le Tourneau T, Markwald RR, Mérot J, Messas E, Milan DP, Neri T, Norris RA, Peal D, Perrocheau M, Probst V, Pucéat M, Rosenthal N, Solis J, Schott JJ, Schwammenthal E, Slaugenhaupt SA, Song JK, Yacoub MH; Leducq Mitral Transatlantic Network . Mitral valve disease—morphology and mechanisms. Nat Rev Cardiol 2015;12:689–710. - PMC - PubMed
-
- Guicciardi NA, De Bonis M, Di Resta C, Ascione G, Alfieri O, Maisano F, Vergara P. Genetic background of mitral valve prolapse. Rev Cardiovasc Med 2022;23:96. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

