Efficacy and safety of cardiac myosin inhibitors for symptomatic hypertrophic cardiomyopathy: a meta-analysis of randomized controlled trials
- PMID: 39882317
- PMCID: PMC11776027
- DOI: 10.3389/fcvm.2024.1477487
Efficacy and safety of cardiac myosin inhibitors for symptomatic hypertrophic cardiomyopathy: a meta-analysis of randomized controlled trials
Abstract
Introduction: Hypertrophic cardiomyopathy (HCM) is a common genetic heart disorder. It is characterized by left ventricular hypertrophy and impaired cardiac function, with forms categorized into obstructive (oHCM) and nonobstructive (nHCM). Traditional treatments address symptoms but not the underlying disease mechanism, highlighting the need for novel therapies. Cardiac myosin inhibitors such as mavacamten and aficamten present potential new treatment options.
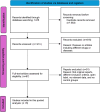
Methods: A meta-analysis of randomized controlled trials (RCTs) was conducted following PRISMA guidelines. Studies comparing cardiac myosin inhibitors with placebo were reviewed, and outcomes related to NYHA functional class, Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS), LVOT gradients, and left ventricular ejection fraction (LVEF) were analyzed.
Results: Six RCTs involving 826 participants demonstrated that mavacamten and aficamten significantly improved NYHA functional class and KCCQ-CSS scores. The incidence of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs) was similar between the treatment and control groups, indicating a comparable safety profile.
Conclusion: Cardiac myosin inhibitors are effective in improving cardiac function and reducing LVOT obstruction in HCM patients. They offer a promising alternative to current treatments, with a safety profile comparable to placebo. Further research is needed to confirm long-term benefits.
Keywords: LVOT obstruction; aficamten; cardiac myosin inhibitors; hypertrophic cardiomyopathy; mavacamten.
© 2025 Abunada, Shah, Kumar, Lamiya Mir, Kumar, Ahmed, Tanzeel, Kumar, Meghjiani, Siddiqui, Khatri, Rai, Deepak and Kumar.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








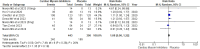
References
-
- Zamorano JL, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. (2014) 35(39):2733–79. 10.1093/eurheartj/ehu284 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

