Value contribution of leniolisib in the Treatment of Activated PI3Kδ syndrome (APDS) in Spain using Multi-Criteria Decision Analysis (MCDA)
- PMID: 39882388
- PMCID: PMC11776101
- DOI: 10.33393/grhta.2025.3199
Value contribution of leniolisib in the Treatment of Activated PI3Kδ syndrome (APDS) in Spain using Multi-Criteria Decision Analysis (MCDA)
Abstract
Background: Activated phosphoinositide 3-kinase (PI3K) δ Syndrome (APDS) is an ultra-rare, potentially life-threatening disease that lacks approved treatments in Spain. This study aimed to apply Multi-Criteria Decision Analysis (MCDA) to assess the value of the first pharmacological treatment for APDS in Spain.
Methods: A multidisciplinary group of 8 experts evaluated the selective PI3Kδ inhibitor leniolisib against Standard of Care (SoC). An MCDA framework tailored for Orphan Drugs (ODs), consisting of 5 comparative and 2 quantitative non-comparative criteria, was used. Re-scoring followed a group discussion.
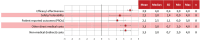
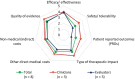
Results: Leniolisib scored higher than SoC in all criteria, including efficacy and safety. It was deemed highly valuable as the first disease-modifying treatment, with a positive therapeutic impact and potential to improve patients' quality of life. Additionally, leniolisib may lead to cost savings. The supporting data was considered of high quality.
Conclusion: Based on MCDA methodology and stakeholder experience in APDS management, leniolisib is seen as a value-added treatment option compared to SoC in Spain.
Keywords: Activated phosphoinositide 3-kinase (PI3K) δ Syndrome (APDS); Decision-making; Multi-criteria Decision Analysis (MCDA); Ultra-rare disease; leniolisib.
© 2025 The Authors.
Conflict of interest statement
Conflict of interest: RA, CA, LIG, AH, ON, CP, CR-G, and JLT have received honoraria from Pharming for their participation in this study. KHH and RF are employees of Pharming. AG is an employee of Omakase Consulting, which received funding from Pharming for the development and performance of this study and the elaboration of the manuscript. None of the authors have received honoraria for the review of the manuscript.
Figures
References
-
- Orphanet: Síndrome de PI3K delta activado. [Accessed July; 2024 ]; Online
LinkOut - more resources
Full Text Sources