First-line treatment for advanced or metastatic EGFR mutation-positive non-squamous non-small cell lung cancer: a network meta-analysis
- PMID: 39882445
- PMCID: PMC11774708
- DOI: 10.3389/fonc.2024.1498518
First-line treatment for advanced or metastatic EGFR mutation-positive non-squamous non-small cell lung cancer: a network meta-analysis
Abstract
Background: Several head-to-head meta-analyses have compared the efficacy and safety of different first-line treatments in patients with EGFR mutation-positive (M+) advanced or metastatic non-squamous non-small cell lung cancer (nsq-NSCLC). However, there is a lack of comprehensive evaluation encompassing multiple treatment strategies. Our objective is to conduct a network meta-analysis that includes various treatment modalities, enabling both direct and indirect comparisons for a more thorough assessment.
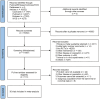
Methods: We conducted a search of PubMed, Embase, Cochrane Library, and Web of Science databases from inception until May 8, 2024, to identify eligible randomized controlled trials (RCTs). The primary endpoints were progression-free survival (PFS) and overall survival (OS), while secondary outcomes included objective response rate (ORR) and grade 3 or higher adverse events (≥3AEs). Stata 15.0 and R 4.3.2 software were utilized for the network meta-analysis.
Results: A total of 30 RCTs, comprising 8654 participants, were included. The study encompassed the following 19 treatments: Chemotherapy; Afatinib; Afatinib + Cetuximab; Apatinib + Gefitinib; Befotertinib; Cetuximab + Chemotherapy; Erlotinib; Erlotinib + Bevacizumab; Erlotinib + Chemotherapy; Gefitinib; Gefitinib + Chemotherapy; Gefitinib + Olaparib; Icotinib; Icotinib + Chemotherapy; Lazertinib; Naquotinib; Osimertinib; Osimertinib + Bevacizumab; Osimertinib + Chemotherapy. The network meta-analysis results indicated that, in terms of PFS, Osimertinib + Chemotherapy (SUCRAs: 93.4%) and Osimertinib (SUCRAs: 84.61%) were the most effective. Regarding OS, Lazertinib (SUCRAs: 89.72%), Gefitinib (SUCRAs: 72.07%), and Osimertinib + Chemotherapy (SUCRAs: 70.74%) emerged as the top three options. Afatinib (SUCRAs: 92.27%) was associated with the best ORR improvement. For ≥3AEs, Afatinib (SUCRAs: 74.93%) and Osimertinib (SUCRAs: 69.42%) were likely the best choices.
Conclusion: Current evidence suggests that, considering both survival and safety, Osimertinib stands out as the preferred first-line treatment for untreated EGFR M + advanced or metastatic nsq-NSCLC. Notably, the combination of Osimertinib with chemotherapy demonstrated superior survival benefits. However, due to the limitations in the number and quality of included studies, these conclusions await further validation through more high-quality research.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42024562981, identifier CRD42024562981.
Keywords: EGFR mutation-positive; first-line treatment; network meta-analysis; non-small cell lung cancer; non-squamous.
Copyright © 2025 Zhang and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Chouaid C, Bensimon L, Clay E, Millier A, Levy-Bachelot L, Huang M, et al. Cost-effectiveness analysis of pembrolizumab versus standard-of-care chemotherapy for first-line treatment of PD-L1 positive (>50%) metastatic squamous and non-squamous non-small cell lung cancer in France. Lung Cancer. (2019) 127:44–52. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

