Natural biomolecules for cell-interface engineering
- PMID: 39882561
- PMCID: PMC11773181
- DOI: 10.1039/d4sc08422e
Natural biomolecules for cell-interface engineering
Abstract
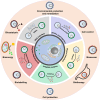
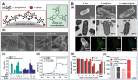
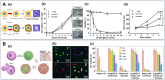
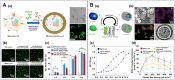
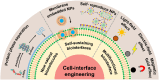
Cell-interface engineering is a way to functionalize cells through direct or indirect self-assembly of functional materials around the cells, showing an enhancement to cell functions. Among the materials used in cell-interface engineering, natural biomolecules play pivotal roles in the study of biological interfaces, given that they have good advantages such as biocompatibility and rich functional groups. In this review, we summarize and overview the development of studies of natural biomolecules that have been used in cell-biointerface engineering and then review the five main types of biomolecules used in constructing biointerfaces, namely DNA polymers, amino acids, polyphenols, proteins and polysaccharides, to show their applications in green energy, biocatalysis, cell therapy and environmental protection and remediation. Lastly, the current prospects and challenges in this area are presented with potential solutions to solve these problems, which in turn benefits the design of next-generation cell engineering.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures















Similar articles
-
Recent advances in research on biointerfaces: From cell surfaces to artificial interfaces.J Biosci Bioeng. 2022 Mar;133(3):195-207. doi: 10.1016/j.jbiosc.2021.12.004. Epub 2022 Jan 5. J Biosci Bioeng. 2022. PMID: 34998688 Review.
-
Novel polymer biomaterials and interfaces inspired from cell membrane functions.Biochim Biophys Acta. 2011 Mar;1810(3):268-75. doi: 10.1016/j.bbagen.2010.04.008. Epub 2010 May 8. Biochim Biophys Acta. 2011. PMID: 20435095 Review.
-
Molecular Approach to Conjugated Polymers with Biomimetic Properties.Acc Chem Res. 2018 Jul 17;51(7):1581-1589. doi: 10.1021/acs.accounts.7b00596. Epub 2018 Jun 13. Acc Chem Res. 2018. PMID: 29897228
-
Cell-membrane-inspired polymers for constructing biointerfaces with efficient molecular recognition.J Mater Chem B. 2022 May 11;10(18):3397-3419. doi: 10.1039/d2tb00242f. J Mater Chem B. 2022. PMID: 35389394 Review.
-
Supramolecular chemistry at interfaces: host-guest interactions for fabricating multifunctional biointerfaces.Acc Chem Res. 2014 Jul 15;47(7):2106-15. doi: 10.1021/ar500105t. Epub 2014 Apr 25. Acc Chem Res. 2014. PMID: 24766328
Cited by
-
Diatom-Based Artificial Antigen-Presenting Cells: A Novel Approach for Adaptive Immune Modulation.ACS Appl Mater Interfaces. 2025 Jul 2;17(26):37718-37734. doi: 10.1021/acsami.5c07766. Epub 2025 Jun 18. ACS Appl Mater Interfaces. 2025. PMID: 40530806 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

