Biparatopic binding of ISB 1442 to CD38 in trans enables increased cell antibody density and increased avidity
- PMID: 39882744
- PMCID: PMC11784651
- DOI: 10.1080/19420862.2025.2457471
Biparatopic binding of ISB 1442 to CD38 in trans enables increased cell antibody density and increased avidity
Abstract
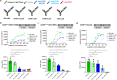
ISB 1442 is a bispecific biparatopic antibody in clinical development to treat hematological malignancies. It consists of two adjacent anti-CD38 arms targeting non-overlapping epitopes that preferentially drive binding to tumor cells and a low-affinity anti-CD47 arm to enable avidity-induced blocking of proximal CD47 receptors. We previously reported the pharmacology of ISB 1442, designed to reestablish synthetic immunity in CD38+ hematological malignancies. Here, we describe the discovery, optimization and characterization of the ISB 1442 antigen binding fragment (Fab) arms, their assembly to 2 + 1 format, and present the high-resolution co-crystal structures of the two anti-CD38 Fabs, in complex with CD38. This, with biophysical and functional assays, elucidated the underlying mechanism of action of ISB 1442. In solution phase, ISB 1442 forms a 2:2 complex with CD38 as determined by size-exclusion chromatography with multi-angle light scattering and electron microscopy. The predicted antibody-antigen stoichiometries at different CD38 surface densities were experimentally validated by surface plasmon resonance and cell binding assays. The specific design and structural features of ISB 1442 enable: 1) enhanced trans binding to adjacent CD38 molecules to increase Fc density at the cancer cell surface; 2) prevention of avid cis binding to monomeric CD38 to minimize blockade by soluble shed CD38; and 3) greater binding avidity, with a slower off-rate at high CD38 density, for increased specificity. The superior CD38 targeting of ISB 1442, at both high and low receptor densities, by its biparatopic design, will enhance proximal CD47 blockade and thus counteract a major tumor escape mechanism in multiple myeloma patients.
Keywords: Biparatopic bispecific antibody; CD38; CD47; CDC; co-crystal structures; innate cell modulator.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures



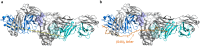




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
